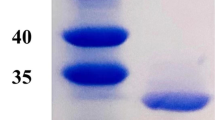
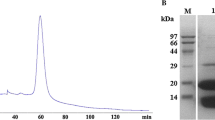
Abstract
We succeeded in homogeneously expressing and purifying l-asparaginase from Latilactobacillus sakei LK-145 (Ls-Asn1) and its mutated enzymes C196S, C264S, C290S, C196S/C264S, C196S/C290S, C264S/C290S, and C196S/C264S/C290S-Ls-Asn1. Enzymological studies using purified enzymes revealed that all cysteine residues of Ls-Asn1 were found to affect the catalytic activity of Ls-Asn1 to varying degrees. The mutation of Cys196 did not affect the specific activity, but the mutation of Cys264, even a single mutation, significantly decreased the specific activity. Furthermore, C264S/C290S- and C196S/C264S/C290S-Ls-Asn1 almost completely lost their activity, suggesting that C290 cooperates with C264 to influence the catalytic activity of Ls-Asn1. The detailed enzymatic properties of three single-mutated enzymes (C196S, C264S, and C290S-Ls-Asn1) were investigated for comparison with Ls-Asn1. We found that only C196S-Ls-Asn1 has almost the same enzymatic properties as that of Ls-Asn1 except for its increased stability for thermal, pH, and the metals NaCl, KCl, CaCl2, and FeCl2. We measured the growth inhibitory effect of Ls-Asn1 and C196S-Ls-Asn1 on Jurkat cells, a human T-cell acute lymphoblastic leukemia cell line, using l-asparaginase from Escherichia coli K-12 as a reference. Only C196S-Ls-Asn1 effectively and selectively inhibited the growth of Jurkat T-cell leukemia, which suggested that it exhibited antileukemic activity. Furthermore, based on alignment, phylogenetic tree analysis, and structural modeling, we also proposed that Ls-Asn1 is a so-called “Type IIb” novel type of asparaginase that is distinct from previously reported type I or type II asparaginases. Based on the above results, Ls-Asn1 is expected to be useful as a new leukemia therapeutic agent.





Similar content being viewed by others
References
Aishwarya SS, Selvarajan E, Iyappan S, Rajnish KN (2019) Recombinant l-asparaginase II from Lactobacillus casei subsp. casei ATCC 393 and its anticancer activity. Ind J.microbiol 59:313–320
Akdel M, Pires DE, Pardo EP, Jänes J, Zalevsky AO, Mészáros B, Bryant P, Good LL, Laskowski RA, Pozzati G, Shenoy A, Zhu W, Kundrotas P, Serra VR, Rodrigues CH, Dunham AS, Burke D, Borkakoti N, Velankar S, Frost A, Basquin J, Lindorff-Larsen K, Bateman A, Kajava AV, Valencia A, Ovchinnikov S, Durairaj J, Ascher DB, Thornton JM, Davey NE, Stein A, Elofsson A, Croll TI, Beltrao P (2022) A structural biology community assessment of alphafold2 applications. Nat Struct Mol Biol 29(11):1056–1067
Bansal S, Srivastava A, Mukherjee G, Pandey R, Verma AK, Mishra P, Kundu B (2012) Hyperthermophilic asparaginase mutants with enhanced substrate affinity and antineoplastic activity: structural insights on their mechanism of action. FASEB J 26(3):1161–1171
Borek D, Kozak M, Pei J, Jaskolski M (2014) Crystal structure of active site mutant of antileukemic l-asparaginase reveals conserved zinc-binding site. FEBS J 281(18):4097–4111
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(248):254
Castro D, Marques ASC, Almeida MR, de Paiva GB, Bento HB, Pedrolli DB, Freire MG, Tavares APM, Santos-Ebinuma VC (2021) l-asparaginase production review: bioprocess design and biochemical characteristics. Appl Microbiol Biotechnol 105:4515–4534
Derst C, Henseling J, Röhm KH (2000) Engineering the substrate specificity of Escherichia coli asparaginase II. selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Sci 9(10):2009–2017
Dübbers A, Würthwein G, Müller HJ, Schulze-Westhoff P, Winkelhorst M, Kurzknabe E, Lanvers C, Pieters R, Kaspers GJ, Creutzig U, Ritter J, Boos J (2000) Asparagine synthetase activity in paediatric acute leukaemias: AML-M5 subtype shows lowest activity. Br J Haematol 109:427–429. https://doi.org/10.1046/j.1365-2141.2000.02015.x
Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, Benoit Y, Robert A, Manel AM, Vilmer E, Otten J, Phillipe N (2002) Comparison of Escherichia coli- asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized european organization for research and treatment of cancer—children’s leukemia group phase 3 trials. Blood 99:2734–2739
Gaufichon L, Rothstein SJ, Suzuki A (2016) Asparagine Metabolic Pathways in Arabidopsis. Plant Cell Physiol 57:675–689. https://doi.org/10.1093/pcp/pcv184
Guo J, Coker AR, Wood SP, Cooper JB, Chohan SM, Rashid N, Akhtar M (2017) Structure and function of the thermostable l-asparaginase from Thermococcus kodakarensis. Acta Crystallogr Sect d Struc Biol 73(11):889–895
Hawkins DS, Park JR, Thomson BG, Holcenberg FJL, JS, Panosyan EH, Avramis VI, (2004) Asparaginase pharmacokinetics after intensive polyethylene glycoL-conjugated l-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin Cancer Res 10:5335–5341. https://doi.org/10.1158/1078-0432.CCR-04-0222
Holm L, Rosenstrom P (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res 38:W545-549
Kato S, Oikawa T (2017) Genome sequence of Lactobacillus sakei LK-145 isolated from a japanese sake cellar as a high producer of d-amino acids. Genome Announc 5:e00656-e717. https://doi.org/10.1128/genomeA.00656-17
Kumar K, Verma N (2012) The various sources and application of l-asparaginase. Asian J Biochem Pharm Res 3:197–205
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lubkowski J, Wlodawer A (2021) Structural and biochemical properties of l-asparaginase. FEBS J 288(14):4183–4209
Maggi M, Chiarelli LR, Valentini G, Scotti C (2015) Engineering of Helicobacter pylori l-asparaginase: characterization of two functionally distinct groups of mutants. PLoS ONE 10(2):e0117025
Maqsood B, Basit A, Khurshid M, Bashir Q (2020) Characterization of a thermostable, allosteric l-asparaginase from Anoxybacillus flavithermus. Int J Biol Macromol 152:584–592
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M (2022) ColabFold: making protein folding accessible to all. Nat Methods 19:679–682
Oikawa T, Okajima K, Yamanaka K, Kato S (2022) First enzymological characterization of selenocysteine β-lyase from a lactic acid bacterium, Leuconostoc mesenteroides. Amino Acids 54:787–798. https://doi.org/10.1007/s00726-022-03133-9
Pedreschi F, Kaack K, Granby K (2008) The effect of asparaginase on acrylamide formation in French fries. Food Chem 109:386–392
Pokrovsky VS, Kazanov MD, Dyakov IN, Pokrovskaya MV, Aleksandrova SS (2016) Comparative immunogenicity and structural analysis of epitopes of different bacterial l-asparaginases. BMC Cancer. https://doi.org/10.1186/s12885-016-2125-4
Ran T, Jiao L, Wang W, Chen J, Chi H, Lu Z, Zhang C, Xu D, Lu F (2020) Structures of l-asparaginase from Bacillus licheniformis reveal an essential residue for its substrate stereoselectivity. J Agric Food Chem 69(1):223–231
Salzer WL, Asselin BL, Plourde PV, Corn T, Hunger SP (2014) Development of asparaginase Erwinia chrysanthemi for the treatment of acute lymphoblastic leukemia. Ann N Y Acad Sci 1329:81–92
Schalk AM, Antansijevic A, Caffrey M, Lavie A (2016) Experimental data in support of a direct displacement mechanism for type I/II l-asparaginases. J Biol Chem 291(10):5088–5100
Sun Z, Qin R, Li D, Ji K, Wang T, Cui Z, Huang Y (2016) A novel bacterial type II l-asparaginase and evaluation of its enzymatic acrylamide reduction in French fries. Int J Biol Macromol 92:232–239
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Yao M, Yasutake Y, Morita H, Tanaka I (2005) Structure of the type i l-asparaginase from the hyperthermophilic archaeon pyrococcus horikoshii at 2 16 a resolution. Acta Crystallogr Sec D Biol Crystallogr 61(3):294–330
Acknowledgements
We thank Ms. Saho Kambe for the construction of pET21-Ls-asn1/Escherichia coli Rosetta (DE3). We thank Ms. Mai Sakata for her fundamental experiments on Ls-Asn1. We thank Ms. Miki Hatanaka for the construction of pET21-C196S, C264S, C290S, C196S/C264S, C196S/C290S, C264S/C290S, and C196S/C264S/C290S-Ls-asn1.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
T. O. designed and supervised the studies. S. K., Y. M., and T. O. wrote the manuscript. K. T. helped the preparation of manuscript partly. K. T., Y. M., and M. K. carried out the experiments. S. K. performed the primary structure, phylogenetic, and modeling analyses. S. K. carried out the molecular docking simulation. K. Y. advised the experiments part.
The authors have not disclosed any funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by PANKAJ BHATT.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kato, S., Tamura, K., Masuda, Y. et al. A novel type IIb l-asparaginase from Latilactobacillus sakei LK-145: characterization and application. Arch Microbiol 206, 266 (2024). https://doi.org/10.1007/s00203-024-03979-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-024-03979-5