Abstract
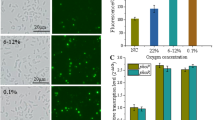
Glyoxylate shunt is an important pathway for microorganisms to survive under multiple stresses. One of its enzymes, malate synthase (encoded by aceB gene), has been widely speculated for its contribution to both the pathogenesis and virulence of various microorganisms. We have previously demonstrated that malate synthase (MS) is required for the growth of Salmonella Typhimurium (S. Typhimurium) under carbon starvation and survival under oxidative stress conditions. The aceB gene is encoded by the acetate operon in S. Typhimurium. We attempted to study the activity of acetate promoter under both the starvation and oxidative stress conditions in a heterologous system. The lac promoter of the pUC19 plasmid was substituted with the putative promoter sequence of the acetate operon of S. Typhimurium upstream to the lacZ gene and transformed the vector construct into E. coli NEBα cells. The transformed cells were subjected to the stress conditions mentioned above. We observed a fourfold increase in the β-galactosidase activity in these cells resulting from the upregulation of the lacZ gene in the stationary phase of cell growth (nutrient deprived) as compared to the mid-log phase. Following exposure of stationary phase cells to hypochlorite-induced oxidative stress, we further observed a 1.6-fold increase in β galactosidase activity. These data suggest the induction of promoter activity of the acetate operon under carbon starvation and oxidative stress conditions. Thus, these observations corroborate our previous findings regarding the upregulation of aceB expression under stressful environments.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Ahn S, Jung J, Jang IA, Madsen EL, Park W (2016) Role of glyoxylate shunt in oxidative stress response. Biol Chem 291(22):11928–11938
Blazeck J, Alper HS (2013) Promoter engineering: recent advances in controlling transcription at the most fundamental level. Biotechnol J 8(1):46–58
Chung T, Resnik E, Stueland C, LaPorte DC (1993) Relative expression of the products of glyoxylate bypass operon: contributions of transcription and translation. J Bacteriol 175:4572–4575
Cozzone AJ (1998) Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Ann Rev Microbiol 52(1):127–164
Cozzone AJ, El-Mansi M (2005) Control of isocitrate dehydrogenase catalytic activity by protein phosphorylation in Escherichia coli. J Mol Microbiol Biotechnol 9:132–146
Dunn MF, Ramirez-Trujillo JA, Hernández-Lucas I (2009) Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiol 155(10):3166–3317
Havis S, Bodunrin A, Rangel J, Zimmerer R, Murphy J, Storey JD, Duong TD, Mistretta B, Gunaratne P, Widger WR, Bark SJ (2019) A universal stress protein that controls bacterial stress survival in Micrococcus luteus. J Bacteriol 201(24):10–128
Jensen PR, Hammer K (1998) The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl Envl Microbiol 64(1):82–87
Jeon JM, Lee HI, Sadowsky MJ, Sugawara M, Chang WS (2015) Characterization of a functional role of the Bradyrhizobium japonicum isocitrate lyase in desiccation tolerance. Int J Mol Sci 16:16695–16709
Jha RK, Kern TL, Fox DT, Strauss M, CE, (2014) Engineering an Acinetobacter regulon for biosensing and high-throughput enzyme screening in E. coli via flow cytometry. Nuc Acd Res 42(12):8150–8160
Jin LQ, Jin WR, Ma ZC, Shen Q, Cai X, Liu ZQ, Zheng YG (2019) Promoter engineering strategies for the overproduction of valuable metabolites in microbes. Appl Microbiol Biotechnol 103:8725–8736
Kim JS, Liu L, Davenport B, Kant S, Morrison TE, Vazquez-Torres A (2022) Oxidative stress activates transcription of Salmonella pathogenicity island-2 genes in macrophages. J Biol Chem 298(7):1021300
Koedooder C, Guéneuguès A, Van Geersdaële R, Vergé V, Bouget FY, Labreuche Y, Obernosterer I, Blain S (2018) The role of the glyoxylate shunt in the acclimation to iron limitation in marine heterotrophic bacteria. Front Mar Sci 5:435
Kornberg HL, Krebs HA (1957) Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature 179:988–991
Koskenkorva T, Frey AD, Kallio PT (2006) Characterization of heterologous hemoglobin and flavohemoglobin promoter regulation in Escherichia coli. J Biotechnol 122(2):161–175
Krebs HA, Johnson WA (1937) The role of citric acid in intermediate metabolism in animal tissues. Enzymologia 4:148–156
Lara AR, Jaén KE, Sigala JC, Mühlmann M, Regestein L, Büchs J (2017) Characterization of endogenous and reduced promoters for oxygen-limited processes using Escherichia coli. ACS Synth Biol 6(2):344–356
Lorenz MC, Fink GR (2002) Life and death in a macrophage: role of the glyoxylate cycle in virulence. Euk Cell 1(5):657–662
Maloy SR, Bohlander M, Nuun WD (1980) Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J Bacteriol 143:720–725
Masayuki Y, Motoki K, Toshio M, Reiko S, Yuji H, Tadayuki I (1991) Promoter sequence analysis in Bacillus and Escherichia: construction of strong promoters in E. coli. Gene 99(1):109–114
Noster J, Chao TC, Sander N, Schulte M, Reuter T, Hansmeier N, Hensel M (2019) Proteomics of intracellular Salmonella enterica reveals roles of Salmonella pathogenicity island 2 in metabolism and antioxidant defense. PLoS Pathog 15(4):e1007741
Priyadarsini S, Panda S, Pashupathi M, Kumar A, Singh R (2021) Design of multiepitope vaccine construct against non-typhoidal Salmonellosis and its characterization using immunoinformatics approach. Int J Pept Res Ther 27:2333–2348
Renilla S, Bernal V, Fuhrer T, Castaño-Cerezo S, Pastor JM, Iborra JL, Sauer U, Cánovas M (2012) Acetate scavenging activity in Escherichia coli: interplay of acetyl–CoA synthetase and the PEP–glyoxylate cycle in chemostat cultures. Appl Microbiol Biotechnol 93:2109–2124
Sarkhel R, Apoorva S, Priyadarsini S, Sridhar HB, Bhure SK, Mahawar M (2022) Malate synthase contributes to the survival of Salmonella Typhimurium against nutrient and oxidative stress conditions. Sci Rep 12(1):15979
Serafini A, Tan L, Horswell S, Howell S, Greenwood DJ, Hunt DM et al (2019) Mycobacterium tuberculosis requires glyoxylate shunt and reverse methylcitrate cycle for lactate and pyruvate metabolism. Mol Microbiol 112(4):1284–1307
Swagatika P, Nikhil KC, Barkha R, Pashupathi M, Parthasarathi B, Ajay K (2019) Effect of mutation resulted from error prone PCR on the strength of promoter activity. J Exptl Zool India 22(2):981–986
Van Acker H, Coenye T (2017) The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol 25:456–466
Wang Y, Wang H, Wei L, Li S, Liu L, Wang X (2020) Synthetic promoter design in Escherichia coli based on a deep generative network. Nuc Acd Res 48(12):6403–6412
Zheng J, Yates SP, Jia Z (2012) Structural and mechanistic insights into the bifunctional enzyme isocitrate dehydrogenase kinase/phosphatase AceK. Philos Trans R Soc B Biol Sci 367(1602):2656–2668
Acknowledgements
RS and SP acknowledge the fellowship support from ICMR and CSIR, India, respectively. The authors thank the Director, ICAR-Indian Veterinary Research Institute for providing the necessary facilities to conduct this study.
Funding
This piece of work was funded by the Department of Biotechnology, India (Grant No.: BT/PR13689/BRB/10/1399/2015) and NASF, ICAR, India (Grant No.: NFBSFARA/BS-3012/2012–13). The funding agency has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
RS and SP contributed equally to this study by performing the experiments, analyzing data, and writing and editing the manuscript. MM reviewed and edited the final draft and supervised the project. RS and SP: Conceptualization, methodology, writing, editing, and revision. MM performed conceptualization, fund acquisition, editing, and revision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
No animals were used for this study.
Consent to participate
No human subjects were used for this study.
Consent to publish
Not applicable.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkhel, R., Priyadarsini, S. & Mahawar, M. Nutrient limitation and oxidative stress induce the promoter of acetate operon in Salmonella Typhimurium. Arch Microbiol 206, 126 (2024). https://doi.org/10.1007/s00203-024-03863-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-024-03863-2