Abstract
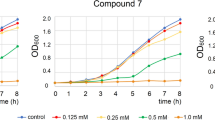
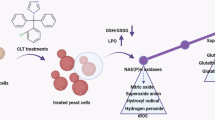
Chalcones have a variety of cellular protective and regulatory functions that may have therapeutic potential in many diseases. In addition, they are considered to affect key metabolic processes in pathogens. Nevertheless, our current knowledge of the action of these compounds against fungal cell is scarce. Therefore, in this study, various substituted chalcone Schiff bases were investigated to reveal their cellular targets within the yeasts Saccharomyces cerevisiae and Candida albicans. First, their antifungal activities were determined via minimum inhibitory concentration method. Surprisingly, parent chalcone Schiff bases showed little or no antifungal activity, while the nitro-substituted derivatives were found to be highly active against yeast cells. Next, we set out to determine the cellular target of active compounds and tested the involvement of the cell wall and cell membrane in this process. Our conductivity assay confirmed that the yeast cell membrane was compromised, and that ion leakage occurred upon treatment with nitro-substituted chalcone Schiff bases. Therefore, the cell membrane came to the fore as a possible target for the active chalcone derivatives. We also showed that exogenous ergosterol added to the growth medium reduced the inhibitory effect of chalcones. Our findings open up new possibilities for the design of future antimicrobial agents based on this appealing backbone structure.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aksöz BE, Rahmiye E (2011) Chemical and structural properties of chalcones I. FABAD J Pharma Sci 36:223–242
Aksöz BE, Onurdağ FK, Özgacar SÖ (2015) Antibacterial, antifungal and antimycobacterial activities of some pyrazoline, hydrazone and chalcone derivatives. Z Naturforsch 70:183–189
Andrade JM, Estevez-Perez MG (2014) Statistical comparison of the slopes of two regression lines: a tutorial. Analytica Chim Acta 838:1–12
Andrade JT, Santos FRS, Lima WG, Sousa CDF, Oliveira LSFM, Ribeiro RIMA, Gomes AJPS, Araújo MGF, Villar JAFP, Ferreira JMS (2018) Design, synthesis, biological activity and structure-activity relationship studies of chalcone derivatives as potential anti-Candida agents. J Antibiot 71:702–712
Ávila HP, Smânia EFA, Monache FD, Smânia Júnior A (2008) Structure–activity relationship of antibacterial chalcones. Bioorg Med Chem 16:9790–9794
Baginski M, Sternal K, Czub J, Borowski E (2005) Molecular modelling of membrane activity of amphotericin B, a polyene macrolide antifungal antibiotic. Acta Biochim Pol 52:655–658
Brajtburg J, Powderly WG, Kobayashi GS, Medoff G (1990) Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother 34:183–188
Chavan PS, Tupe SG (2014) Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control 46:115–120
Dimmock JR, Elias DW, Beazely MA, Kandepu NM (1999) Bioactivities of chalcones. Curr Med Chem 6:1125–1149
Ellis D (2002) Amphotericin B: spectrum and resistance. J Antimicrob Chemother 49:7–10
Ergüden B, Ünver Y (2022) Phenolic chalcones lead to ion leakage from Gram-positive bacteria prior to cell death. Arch Microbiol 204(1):1–6
Hammer KA, Carson CF, Riley TV (2004) Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimic Chemother 53(6):1081–1085
Hammoudi HD, Younes S, Mourad N, Rahal M (2022) Allylamines, benzylamines, and fungal cell permeability: a review of mechanistic effects and usefulness against fungal pathogens. Membranes 12(12):1171
Hasibagan B, Akira O, Ken-ichi F, Eiji H, Toshio T (2009) The vacuole-targeting fungicidal activity of Amphotericin B against the pathogenic fungus candida albicans and its enhancement by allicin. J Antibiot 62:691–697
Hossain CM, Ryan LK, Gera M, Choudhuri S, Lyle N, Ali KA, Diamond G (2022) Antifungals and drug resistance. Encyclopedia 2(4):1722–1737
Ivanov M, Ćirić A, Stojković D (2022) Emerging antifungal targets and strategies. Int J Mol Sci 23(5):2756
Konuk H, Ergüden B (2020) Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol 65(4):775–783
Mahapatra DK, Bharti SK, Asati V (2015) Chalcone scaffolds as anti-infective agents: structural and molecular target perspectives. Eur J Med Chem 101:496–524
Mani-López E, Cortés-Zavaleta O, López-Malo A (2021) A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl Sci 3(1):1–25
Masuda A, Akiyama SI, Kuwano M (1982) Potentiation of antifungal effect of Amphotericin B by squalene, an intermediate for sterol biosynthesis. J Antibiot 35:230–234
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcone. Eur J Med Chem 42:125–137
Pereira FO, Mendes JM, Lima EO (2013) Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med Mycol 51:507–513
Reddy GKK, Padmavathi AR, Nancharaiah YV (2022) Fungal infections: pathogenesis, antifungals and alternate treatment approaches. Curr Res Microbial Sci 3:100137
Rodrigues MC, Araujo WAF, de Oliveira IM, Lana DFD, Carneiro GKM (2022) Resistance of filamental fungi in opportunistic mycoses: literature review. Res Soc Dev 11:e2011426198
Sant DG, Tupe SG, Ramana CV, Deshpande MV (2016) Fungal cell membrane—promising drug target for antifungal therapy. J Appl Microbiol 121(6):1498–1510
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:9–27
Silva WA, Andrade CKZ, Napolitano HB, Vencato I, Lariucci C, de Castrob MRC, Camargob AJ (2013) Biological and structure-activity evaluation of chalcone derivatives against bacteria and fungi. J Braz Chem Soc 24:33–144
Sivakumar PM, Priya S, Doble M (2009) Synthesis, biological evaluation, mechanism of action and quantitative structure-activity relationship studies of chalcones as antibacterial agents. Chem Biol Drug Des 73:403–415
Tekale S, Mashele S, Pooe O, Thore S, Kendrekar P, Pawar R (2020) Biological role of chalcones in medicinal chemistry. Vector-borne diseases-recent developments in epidemiology and control. IntechOpen, London, pp 117–134
Ünver Y, Tuluk M, Kahriman N, Emirik M, Bektaş E, Direkel Ş (2019) New chalcone derivatives with schiff base-thiophene: synthesis, biological activity, and molecular docking studies. Russian J Gen Chem 89:794–799
Wang Y, Zeng X, Zhou Z, Xing K, Tessema A, Zeng H, Tian J (2015) Inhibitory effect of nerol against Aspergillus niger on grapes through a membrane lesion mechanism. Food Control 55:54–61
Zhang YQ, Gamarra S, Garcia-Effron G, Park S, Perlin DS, Rao R (2010) Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog 6:e1000939
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117:7762–7810
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
YÜ synthesized the compounds 1–8, HBL and BE designed and performed the experiments. YÜ, HBL and BE wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ergüden, B., Lüleci, H.B. & Ünver, Y. Chalcone Schiff bases disrupt cell membrane integrity of Saccharomyces cerevisiae and Candida albicans cells. Arch Microbiol 205, 246 (2023). https://doi.org/10.1007/s00203-023-03584-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03584-y