Abstract
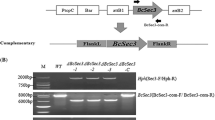
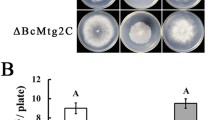
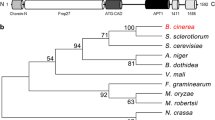
Botrytis cinerea is a non-host-specific phytopathogenic fungus capable of infecting numerous cash crops. Here, we analyzed the functions of the Bcb1 gene in B. cinerea, which encodes a membrane protein belonging to the acyl-coenzyme A synthase family. Compared to the wild type, Bcb1-deletion mutants exhibited obvious morphological abnormalities, including slower vegetative growth and reduced melanin production. The absence of Bcb1 causes B. cinerea to form only small and incompletely developed infection cushions and fail to produce spores. The Bcb1 mutants displayed hypersensitivity to the membrane stressor SDS, the cell wall stressor Congo red, and the oxidative stressor H2O2 and increased resistance to intracellular osmotic stress caused by KCl compared to the wild-type strain. However, there were no differences in tolerance to extracellular osmotic stress caused by NaCl. The deletion of Bcb1 also caused a reduction in pathogenicity. The qRT‒PCR results showed that the genes Bcpks12 and Bcpks13, which are related to melanin biosynthesis, and Bcpg2, BcBOT2, and cutA, which are related to virulence, were downregulated in ∆Bcb1. These data suggest that BCB1 is important for conidial morphogenesis, and pathogenesis in B. cinerea.




Similar content being viewed by others
References
An B, Li B, Li H et al (2016) Aquaporin8 regulates cellular development and reactive oxygen species production, a critical component of virulence in Botrytis cinerea. New Phytol 209:1668–1680
Backhouse D, Willetts HJ (1987) Development and structure of infection cushions of Botrytis cinerea. Trans Br Mycol 89:89–95
Black PN, DiRusso CC (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta 1771:286–298
Bulawa CE (1993) Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol 47:505–534
Carisse O, McNealis V, Kriss A (2018) Association between weather variables, airborne inoculum concentration and raspberry fruit rot caused by Botrytis cinerea. Phytopathology 108:70–82
Catlett NL, Lee B, Yoder OC et al (2003) Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet Rep 50:Article 4
Chen X, Zhu C, Na Y et al (2021) Compartmentalization of melanin biosynthetic enzymes contributes to self-defense against intermediate compound scytalone in Botrytis cinerea. Mbio 12:e00007-21
Choquer M, Rascle C, Gonçalves IR et al (2021) The infection cushion of Botrytis cinerea: a fungal “weapon” of plant-biomass destruction. Environ Microbiol 23:2293–2314
Feng HQ, Li GH, Du SW et al (2017) The septin protein Sep4 facilitates host infection by plant fungal pathogens via mediating initiation of infection structure formation. Environ Microbiol 19:1730–1749
Grevengoed TJ, Klett EL, Coleman RA (2014) Acyl-CoA metabolism and partitioning. Annu Rev Nutr 34:1–30
Have AT, Mulder W, Visser J et al (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11:1009–1016
Jackson DR, Tu SS, Nguyen M, Barajas JF, Schaub AJ, Krug D, Pistorius D, Luo R, Müller R, Tsai SC (2016) Structural insights into anthranilate priming during type II polyketide biosynthesis. ACS Chem Biol 11(1):95–103
Jia SL, Chi Z, Chen L, Liu GL, Hu Z, Chi ZM (2021) Molecular evolution and regulation of DHN melanin-related gene clusters are closely related to adaptation of different melanin-producing fungi. Genomics 113(4):1962–1975
Jiang Y, Wang W, Xie Q et al (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356:1172–1175
Jiménez-Munguía I, Volynsky PE, Batishchev OV et al (2019) Effects of sterols on the interaction of SDS, benzalkonium chloride, and a novel compound, kor105, with membranes. Biomolecules 9:627
Jin M, Yang C, Wei L, Cui L et al (2021) First report of Botrytis cinerea causing gray mold on Astragalus membranaceus in China. Plant Dis
Kars I, Krooshof GH, Wagemakers L et al (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J 43:213–125
Li XH, Peng YJ, Ding JL et al (2022) A homologue of yeast acyl-CoA synthetase Faa1 contributes to cytomembrane functionality involved in development and virulence in the insect pathogenic fungus Beauveria bassiana. Microb Pathog 164:105419
Liu QL, Zhang JX, Xu RF et al (2007) The influence aspect on using cellulose culture medium containing congo red differential medium to screen microorganism producing celluse. Acta Agric Boreali Occident Sin 16:279–281
Liu X, Xie J, Fu Y et al (2020) The subtilisin-Like protease Bcser2 affects the sclerotial formation, conidiation and virulence of Botrytis cinerea. Int J Mol Sci 21:603
Livak KJ, Schmittgen TD (2001)Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lu SW, Kroken S, Lee BN et al (2003) A novel class of gene controlling virulence in plant pathogenic ascomycete fungi. Proc Natl Acad Sci USA 100:5980–5985
Ma Z, Chen Z, Wang W et al (2020) Exocyst subunit BcSec3 regulates growth, development and pathogenicity in Botrytis cinerea. J Biosci 45:125
Michielse CB, Becker M, Heller J et al (2011) The Botrytis cinerea Reg1 protein, a putative transcriptional regulator, is required for pathogenicity, conidiogenesis, and the production of secondary metabolites. Mol Plant Microbe Interact 24:1074–1085
Moretti C, Quaglia M, Cerri M et al (2015) A real-time PCR assay for detection and quantification of Botrytis cinerea in Pelargonium × hortorum plants and its use for evaluation of plant resistance. Eur J Plant Pathol 143:159–171
Petrasch S, Knapp SJ, van Kan JAL et al (2019) Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol Plant Pathol 20:877–892
Pinedo C, Wang CM, Pradier JM et al (2008) Sesquiterpene synthase from the botrydial biosynthetic gene cluster of the phytopathogen Botrytis cinerea. ACS Chem Biol 19:791–801
Potisek M, Likar M, Vogel-Mikus K, Arcon I, Grdadolnik J, Regvar M (2021) 1,8-Dihydroxy naphthalene (DHN)-melanin confers tolerance to cadmium in isolates of melanised dark septate endophytes. Ecotoxicol Environ Saf 222:112493
Ram AF, Klis F (2006) Model organisms identification of fungal cell wall mutants using susceptibility assays based on calcofluor white and Congo red. Nat Protoc 1:2253–2256
Schmelz S, Naismith JH (2009) Adenylate-forming enzymes. Curr Opin Struct Biol 19:666–671
Schumacher J (2016) DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol Microbiol 99:729–748
Schumacher J (2017) How light affects the life of Botrytis. Fungal Genet Biol 106:26–41
Segmüller N, Ellendorf U, Tudzynski B et al (2007) BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell 6:211–221
Shao W, Lv C, Zhang Y et al (2017) Involvement of BcElp4 in vegetative development, various environmental stress response and virulence of Botrytis cinerea. Microb Biotechnol 10:886–895
van Kan JA, van't Klooster JW, Wagemakers CA, Dees DC, van der Vlugt-Bergmans CJ (1997) Cutinase A of Botrytis cinerea is expressed, but not essential, during penetration of gerbera and tomato. Mol Plant Microbe Interact 10(1):30–38
Veloso J, van Kan JAL (2018) Many shades of grey in botrytis-host plant interactions. Trends Plant Sci 23:613–622
Wang HC, Li WH, Wang MS et al (2011) First report of Botrytis cinerea causing gray mold of tobacco in Guizhou Province of China. Plant Dis 95:612
Wang X, Xing JH, Zhao B et al (2013) Cloning and functional analysis of a gene related to conidiospore formation in Botrytis cinerea. Microbiol China 40:533–543
Wang Y, He D, Chu Y et al (2016) MoCps1 is important for conidiation, conidial morphology and virulence in Magnaporthe oryzae. Curr Genet 62:861–871
Wang Y, Li G, Chen T, Tian S (2022) Protein sulfenylation contributes to oxidative burst-triggered responses during the interaction between Botrytis cinerea and Nicotiana benthamiana. J Proteom 251:104423
Xue XM (2016) Research of pathogenicity and sclerotia related genes in Botrytis cinerea. Master’s thesis, Huazhong Agricultural University, Wu Han
Yu QY (2017) Functional analysis of autophagy-related genes BcATG26, BcATG17 and BcATG14 in Botrytis cinerea. Master’s thesis, Huazhong Agricultural University, Wu Han
Yu JR, Zhao SG, Xu YS (2014) First report of gray mold on Amorphophallus muelleri caused by Botrytis cinerea in China. Plant Dis 98:652
Zhang M, Wu HY, Wang XJ (2014) First report of Botrytis cinerea causing Eruit rot of Pyrus sinkiangensis in China. Plant Dis 98:281
Zhang C, He Y, Zhu P et al (2015) Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence. Mol Plant Microbe Interact 28:1091–1101
Zheng HX (2013) Pathogenicity regulatory function of BcPDR1 gene in Botrytis cinerea. Master’s thesis, Heibei agricultural university, Heibei
Acknowledgements
This work was supported by Grants from the National Key R&D Program of China (2019YFD1002002), the Agricultural Science and Technology Innovation Fund of Hunan Province (2022CX72), the National Key Research and Development Program of Hunan (2021NK2003), Hunan Agriculture Research System (2022-31).
Author information
Authors and Affiliations
Contributions
Jiling Xiao and Ke Yang wrote the main manuscript text and Zhihuai Liang performed the data analysis. Yi Zhang and Lin Wei prepared all the figures.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Yang, K., Liang, Z. et al. BCB1, a member of the acyl-coenzyme A synthetase family, regulates the morphogenesis and pathogenicity of Botrytis cinerea. Arch Microbiol 205, 206 (2023). https://doi.org/10.1007/s00203-023-03540-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03540-w