Abstract
The present study aimed to evaluate the potential and specificity of the inflammatory and antioxidant response of Microbe‐Associated Molecular Patterns (MAMPs) in NIH-3T3 fibroblast cells, as well as in the healing process of skin wounds. Cells (NIH-3T3) were cultivated in supplemented specific medium. NIH-3T3 cells were treated with MAMPs (Bifidobacterium lactis or Lactobacillus casei or Lactobacillus gasseri or Lactobacillus paracasei or Streptococcus thermophilus), at two concentrations and insulted with LPS or H2O2. Cell viability, myeloperoxidase activity, nitrite/nitrate, oxidative damage and inflammatory parameters were measured. In addition, scratch assay was performed. Significant scratch closure was observed after 24 h and 48 h, and the effect of 0.1 g/mL MAMPs on wound healing was found to be highly statistically significant. In the viability cellular assay, Lactobacillus showed better response in 0.1 g/mL dose, whereas B. lactis and S. thermophilus showed better response in 0.01 g/mL dose. There was reduction in IL-6 and IL-1β levels in all treatments insulted with LPS. MAMP’s showed preventive efficacy in reducing the effects caused by LPS. The MAMP’s action in decreasing the production of ROS, inflammatory activity and increasing cell viability, besides significant cell proliferation during wound healing processes suggests remodeling mechanisms and new possibilities for wound healing.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adams CA (2010) The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev 23(1):37–46
Ahlina FN, Nugraheni N, Salsabila IA, Haryanti S, Da’i M, Meiyanto E (2020) Revealing the reversal effect of galangal (Alpinia galanga L.) extract against oxidative stress in metastatic breast cancer cells and normal fibroblast cells intended as a Co- chemotherapeutic and anti-ageing agent. Asian Pac J Cancer Prev 21(1):107–117
Bowe WP, Logan AC (2011) Acne vulgaris, probiotics and the gut-brain-skin axis-back to the future? Gut Pathog 3(1):1
Brezoiu AM, Bajenaru L, Berger D, Mitran RA, Deaconu M, Lincu D, Stoica Guzun A, Matei C, Moisescu MG, Negreanu-Pirjol T (2020) Effect of nanoconfinement of polyphenolic extract from grape pomace into functionalized mesoporous silica on its biocompatibility and radical scavenging activity. Antioxid (basel) 9(8):696
Buckley CD (2003) Why does chronic inflammatory joint disease persist? Clin Med 3:361–366
Campos LF, Tagliari E, Casagrande TAC, Noronha L, Campos ACL, Matias JEF (2020) Effects of probiotics supplementation on skin wound healing in diabetic rats. Arq Bras Cir Dig 33(1):e1498
Chen J, Chen Y, Yang Z, You B, Ruan YC, Peng Y (2016) Epidermal CFTR suppresses MAPK/NF-kappaB to promote cutaneous wound healing. Cell Physiol Biochem 39(6):2262–2274
Choi JH, Jun JH, Kim JH, Sung HJ, Lee JH (2014) Synergistic effect of interleukin-6 and hyaluronic acid on cell migration and ERK activation in human keratinocytes. J Korean Med Sci 29:S210–S216
Cinque B, La Torre C, Melchiorre E, Marchesani G, Zoccali G, Palumbo P, Di Marzio L, Masci A, Mosca L, Mastromarino P, Giuliani M, Cifone MG (2011) Use of probiotics for dermal applications. Microbiol Monogr 21:221–241
Coleman SJ, Bruce C, Chioni AM, Kocher HM, Grose RP (2014) The ins and outs of fibroblast growth factor receptor signalling. Clin Sci 127(4):217–231
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–341
Draper HH, Hadley M (1990) Malondialdehyde determination as índex of lipid peroxidation. Methods Enzymol 186:421–431
Eming SA, Wynn TA, Martin P (2017) Inflammation and metabolism in tissue repair and regeneration. Science 356:1026–1030
Español AJ, Maddaleno MO, Lombardi MG, Cella M, Martínez Pulido P, Sales ME (2014) Treatment with LPS plus INF-γ induces the expression and function of muscarinic acetylcholine receptors, modulating NIH3T3 cell proliferation: participation of NOS and COX. Br J Pharmacol 171(22):5154–5167
Gnerucci A, Faraoni P, Sereni E, Ranaldi F (2020) Scratch assay microscopy: a reaction-diffusion equation approach for common instruments and data. Math Biosci 330:108482
Gonzalez AC, Costa TF, Andrade ZA, Medrado AR (2016) Wound healing–a literature review. An Bras Dermatol 91(5):614–620
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and nitrate in biological fluids. Anal Biochem 126:131–138
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321
Haas GS, Wang W, Saffar M, Mooney-Leber SM, Brummelte S (2020) Probiotic treatment (Bifidobacterium longum Subsp. longum 35624™) affects stress responsivity in male rats after chronic corticosterone exposure. Behav Brain Res 393:112718
Hrabak A, Bajor T, Csuka I (2008) The effect of various inflammatory agents on the phagocytosis and cytokine profile of mouse and rat macrophages. Inflammation Res 57(2):75–83
Huseini HF, Rahimzadeh G, Fazeli MR, Mehrazma M, Salehi M (2012) Evaluation of wound healing activities of kefir products. Burns 38:719–723
Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH (2011) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 11:CD004827
Khatun Z, Nishimura N, Kobayashi D, Hazama A (2020) Cesium suppresses fibroblast proliferation and migration. Fukushima J Med Sci 66(2):97–102
Kiousi DE, Karapetsas A, Karolidou K, Panayiotidis MI, Pappa A, Galanis A (2019) Probiotics in extraintestinal diseases: current trends and new directions. Nutrients 11:788
Knackstedt R, Knackstedt T, Gatherwright J (2020) The role of topical probiotics on wound healing: a review of animal and human studies. Int Wound J. https://doi.org/10.1111/iwj.13451
Komarcević A (1999) The modern approach to wound treatment. Med Pregl 53:363–368
Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R (2020) Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol 60(1):12–25
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Li J, Zhang YP, Kirsner RS (2003) Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Micros Res Tech 60:107–114
Liu H, Mu L, Tang J, Shen C, Gao C, Rong M, Zhang Z, Liu J, Wu X, Yu H, Lai R (2014) A potential wound healing-promoting peptide from frog skin. Int J Biochem Cell Biol 49:32–41
Lombardi F, Palumbo P, Mattei A, Augello FR, Cifone MG, Giuliani M, Cinque B (2019) Soluble fraction from lysates of selected probiotic strains differently influences re-epithelialization of HaCaT scratched monolayer through a mechanism involving nitric oxide synthase 2. Biomolecules 9(124):756
Marshall H, Merchant K, Stamler J (2000) Nitrosation and oxidation in the regulation of gene expression. FASEB J 14:1889–1900
Mohajer Ansari J, Ramhormozi P, Shabani R, Pazoki-Toroudi H, Yari A, Barati M, Dahmardehei M, Babakhani A, Nobakht A (2020) Simvastatin combined with bone marrow mesenchymal stromal cells (BMSCs) improve burn wound healing by ameliorating angiogenesis through SDF-1α/CXCR4 pathway. Iran J Basic Med Sci 23(6):751–759
Mohammedsaeed W, Cruickshank S, McBain AJ, O'Neill CA (2015) Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Sci Rep 5:16147. https://doi.org/10.1038/srep16147
Nataraj BH, Ali SA, Behare PV, Yadav H (2020) Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19(1):168
Oeckinghaus A, Ghosh S (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1:a000034
Ouyang QQ, Hu Z, Lin ZP, Quan WY, Deng YF, Li SD, Li PW, Chen Y (2018) Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int J Biol Macromol 112:1191–1198
Park YR, Sultan MT, Park HJ, Lee JM, Ju HW, Lee OJ, Lee DJ, Kaplan DL, Park CH (2018) NF-kappa B signaling is key in the wound healing processes of silk fibroin. Acta Biomater 67:183–195
Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 2000(7):165–197
Rovito HA, Oblong JE (2013) Nicotinamide preferentially protects glycolysis in dermal fibroblasts under oxidative stress conditions. Br J Dermatol 169(Suppl 2):15–24
Szabowski N, Maas-Szabowski S, Andrecht A, Kolbus M, Schorpp-Kistner NE, Fusenig P (2000) Angel. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell 103(5):745–755
Takada K, Komine-Aizawa S, Hirohata N, Trinh QD, Nishina A, Kimura H, Hayakawa S (2017) Poly I: C induces collective migration of HaCaT keratinocytes via IL-8. BMC Immunol 18:2–10
Taverniti V, Guglielmetti S (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6:261–274
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870
Wong VW, Martindale RG, Longaker MT, Gurtner GC (2013) From germ theory to germ therapy: skin microbiota, chronic wounds, and probiotics. Plast Reconstr Surg 132:854e–861e
Xuan Y, Chi L, Tian H, Cai W, Sun C, Wang T, Zhou X, Shao M, Zhu Y, Niu C, Sun Y, Cong W, Zhu Z, Li Z, Wang Y, Jin L (2016) The activation of the NF-κB-JNK pathway is independent of the PI3K-Rac1-JNK pathway involved in the bFGF-regulated human fibroblast cell migration. J Dermatol Sci 82(1):28–37
Yang DJ, Moh SH, Son DH, You S, Kinyua AW, Ko CM, Song M, Yeo J, Choi YH, Kim KW (2016) Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules 21(7):899
Yu Y, Lu J, Oliphant K, Gupta N, Claud K, Lu L (2020) Maternal administration of probiotics promotes gut development in mouse offsprings. PLoS ONE 15(8):e0237182
Acknowledgements
UNESC, Biohall Research and Innovation; Gabbia Biotechnology.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico, MONIQUE MICHELS-PDI.
Author information
Authors and Affiliations
Contributions
MM, GFAJ—conceptualization and design of the study; Data curation; Formal analysis and Methodology; Roles/Writing—original draft. EC, LBGR, RD, APLV, MPR, FR—conceptualization and design of the study; Methodology FDP—conceptualization and design of the study; Project administration; Supervision; Funding acquisition; Writing—review & editing. All authors approved final version submitted. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Corresponding author
Ethics declarations
Conflict of interest
Gabbia Biotechnology are developing paraprobiotics for the commercial purposes. Marina Rosseto, Ana Paula Voytena and Fernanda Ramlov are members of Gabbia Biotechnology. Monique Michels and Gabriel Alves Jesus are members of Biohall Research and Innovation. The authors declared no potential conflicts of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
203_2023_3469_MOESM1_ESM.png
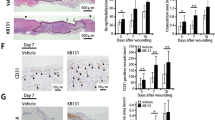
Supplementary file1 NIH-3T3 cells were insulted with LPS or H2O2 that increase ROS generation and cytokines production. After treatment with MAMP’s (paraprobiotics), these parameters decreased. Besides this, re-epithelization occurs after treatment (PNG 625 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michels, M., Córneo, E., Rocha, L.B.G. et al. Paraprobiotics strains accelerate wound repair by stimulating re-epithelialization of NIH-3T3 cells, decreasing inflammatory response and oxidative stress. Arch Microbiol 205, 134 (2023). https://doi.org/10.1007/s00203-023-03469-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03469-0