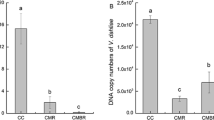
Abstract
The contribution of crops and soil microbial community structure and functional diversity in soil-borne diseases control mulberry plant production is still inadequately understood. In this work, a comparative study was undertaken on the microbial abundance, community structure, and functional diversity in the soil rhizosphere between the resistant (Kangqing 10) and the susceptible (Guisang 12) mulberry genotypes. The study deployed the use of dilution plate method, micro-ecology technology, and polymerase chain reaction–denaturing gradient gel electrophoresis (PCR-DGGE) techniques. The study aimed at developing better crop management methods for mulberry cultivation as well as preventing and controlling the occurrence and impacts of bacterial wilt on mulberry productivity. The results indicated that the soil rhizosphere microorganisms were more abundant in the normal resistant mulberry genotype than in the normal susceptible mulberry genotype. Carbon source utilization was better in the normal susceptible mulberry genotype. These properties were lower in the sickly resistant mulberry genotype than in the susceptible sickly mulberry genotype. Through the PCR–DGGE, it was shown that the bacterial and fungal community structures of the resistant genotypes were more stable than those of the susceptible genotypes. Through correlation regression analysis, it was shown that the mulberry bacterial wilt significantly contributes to the loss of soil nutrients, particularly organic matter and nitrogen, a possible cause to disrupted balance between the soil microbial community and the loss of soil organic matter. Resistant genotype plants displayed more resistance to bacterial wilt. Therefore, this study recommends the need to promote the cultivation of resistant genotype mulberry for increased yield.







Similar content being viewed by others
References
Alebiosu IB, Olatunde GO, Pitan OR (2013) General performance and cocoon yields of two hybrids of the silkworm Bombyx mori L. (Lepidoptera: Bombicidae), fed on mulberry leaves from different amended soils. Ann Trop Res. https://doi.org/10.3294/atr3511.2013
Ashaduzzaman SM, Israt ZM, Li CF, Dai CC (2016) Endophytic fungus Phomopsis liquidambari and different doses of N-fertilizer alter microbial community structure and function in rhizosphere of rice. Sci Rep 6:32270
Ayana G, Fininsa C, Ahmed S, Wydra K (2011) Effects of soil amendment on bacterial wilt caused by Ralstonia solanacerum and tomato yields in Ethiopia. J Plant Protect Res 51(1):72–76
Bao SD (2000) Soil and agricultural chemistry analysis, 3rd edn. China Agricultural Press, Beijing, pp 265–267
Blakemore LC (1987) Methods for chemical analysis of soils. N Z Soil Bur Sci Rep 80:19–22
Bottos EM, Vincent WF, Greer CW, Whyte LG (2008) Prokaryotic diversity of arctic ice shelf microbial mats. Environ Microbiol 10(4):950–966
Cen ZL, Ouyang QF, Xie L, Chen BS, Zhu FR, Lin Q, Qin LP, Meng JR, Shi GY, Zhang WL (2013) Isolation and identification of pathogenic bacteria from mulberry bacterial wilt in Guangxi. Southwest China J Agric Sci 26(3):1054–1057
Charkowski A, Sharma K, Parker ML, Secor GA, Elphinstone J (2020) Bacterial diseases of potato. In: Campos H, Ortiz O (eds) The potato crop. Springer, New York
Chen F, Lu J, Zhang M, Wan K, Liu D (2009) Mulberry nutrient management for silk production in Hubei province of China. J Plant Nutr Soil Sci 172(2):245–253. https://doi.org/10.1002/jpln.200800093
Couëdel A, Alletto L, Kirkegaard J, Justes É (2018) Crucifer glucosinolate production in legume-crucifer cover crop mixtures. Eur J Agron 96:22–33
Dignam BEA, O’Callaghan M, Condron LM, Kowalchuk GA, Nostrand JDV, Zhou J, Wakelin SA (2018) Effect of land use and soil organic matter quality on the structure and function of microbial communities in pastoral soils: implications for disease suppression. PLoS ONE 13(5):e0196581
Edil TB (1988) Soil sampling. In: General geology. Encyclopedia of earth science. Springer, Boston, MA. https://doi.org/10.1007/0-387-30844-X_106
Enwall K, Philippot L, Hallin S (2009) Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Acta Ecol Sin 71(12):8335–8343
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28(2):213–221
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57(8):2351–2359
Ghose L, Neela FA, Chakravorty TC, Ali MR, Alam MS (2010) Incidence of leaf blight disease of mulberry plant and assessment of changes in amino acids and photosynthetic pigments of infected leaf. Plant Pathol J 9(3):140–143
Gopalakrishnan TR, Singh PK, Sheela KB, Shankar MA, Kutty PCJ, Peter KV, Allen C, Prior P, Hayward AC (2005). Development of bacterial wilt resistant varieties and basis of resistance in eggplant (Solanum melongena L.). In: Allen C, Prior P, Hayword A (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex, pp 293–300
Guo X, Chen DD, Peng KS, Cui ZW, Zhang XJ, Li S, Zhang YA (2016) Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol 52:74–84
Hayward AC (2003) Biology and epidemiology of bacterial wilt caused by pseudomonas Solanacearum. Ann Rev Phytopathol 29(1):65–87
Hiromichi Y, Osamu I, Tsuguo H (1996) Relationship between resistance to bacterial wilt and nutrient uptake in tomato seedlings. Soil Sci Plant Nutr 42(1):203–208
Hirte WF (1969). The use of dilution plate method for the determination of soil microflora. 2. The qualitative demonstration of bacteria and actinomycetes. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Zweite naturwissenschaftliche Abt.: Allgemeine, landwirtschaftliche und technische Mikrobiologie 123(2):167
Illera-Vives M, Labandeira SS, Loureiro LI, López-Mosquera ME (2017) Agronomic assessment of a compost consisting of seaweed and fish waste as an organic fertilizer for organic potato crops. J Appl Phycol 29(3):1663–1671
Irikiin Y, Nishiyama M, Otsuka S, Senoo K (2006) Rhizobacterial community-level, sole carbon source utilization pattern affects the delay in the bacterial wilt of tomato grown in rhizobacterial community model system. Appl Soil Ecol 34(1):27–32
Ji X (2008) Biological control against bacterial wilt and colonization of mulberry by an endophytic Bacillus subtilis strain. FEMS Microbiol Ecol 65(3):565–573. https://doi.org/10.1111/j.1574-6941.2008.00543.x
Jia L, Wei R, Jiang H, Yang XM, Xu YC, Shen QR (2010) Application of bio-organic fertilizer significantly affected fungal diversity of soils. Soil Sci Soc Am J 74(6):2039
Kariko EK, Murimi ZK, Owuor PO, Maingi JM (2016) Bacterial wilt, a challenge in Solanaceous crops production at Kenyan highlands and lowlands. World J Res Rev (WJRR) 3(1):6–11
Lit MTC, Opina NL, Lapiz RV (2002) Identification of eggplant varieties resistant to the leafhopper, shoot/fruit borer and bacterial wilt. Paper presented at the 33. Anniversary and Annual Scientific Meeting of the Pest Management Council of the Philippines, Inc., Davao City (Philippines), pp 8–10
Liu X, Lei H, Chen F (2019) Infection pattern and negative effects of a facultative endosymbiont on its insect host are environment-dependent. Sci Rep 9:4013. https://doi.org/10.1038/s41598-019-40607-5
Mahadevakumar S, Chandana C, Deepika YS, Sumashri KS, Yadav V, Janardhana GR (2018) Pathological studies on the southern blight of China aster (Callistephus chinensis) caused by Sclerotium rolfsii. Eur J Plant Pathol 3:1–7
Matsuda T (1977) Fundamental studies on the breeding of bacterial wilt resistant varieties in tobacco. Bull Utsunomiya Tob Exp Stn 15:1–95
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, pp 539–579
Niu J, Chao J, Xiao Y, Chen W, Zhang C, Liu X, Rang Z, Yin H, Dai L (2017) Insight into the effects of different cropping systems on soil bacterial community and tobacco bacterial wilt rate. J Basic Microbiol 57(1):3–11. https://doi.org/10.1002/jobm.201600222
Opina NL, Santiago SE, Tiongco RL, Miller S, Maghirang RG (2001) Influence of cultural practices, host resistance and grafting on the incidence of bacterial wilt on eggplant. Eur Spine J 23(4):754–761
Pinta M (1970) Agricultural applications of flame photometry. Macmillan Education, UK
Riley D, Barber SA (1969) Bicarbonate accumulation and pH changes at the soybean (Glycine max (L.) Merr.) root–soil interface 1. Soil Sci Soc Am J 33(6):905–908
Rui W, Zhang H, Sun L, QiG ShuC, Zhao X (2017) Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7(1):343
She S, Niu J, Chao Z, Xiao Y, Wu C, Dai L, Liu X, Yin H (2017) Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch Microbiol 199(2):1–9
Siah A, Magnin-Robert M, Randoux B, Choma C, Rivière C, Halama P, Reignault P (2018) Natural agents inducing plant resistance against pests and diseases. In: Mérillon JM, Riviere C (eds) Natural antimicrobial agents, vol 19. Springer, New York
Staddon WJ, Duchesne LC, Trevors JT (1997) Microbial diversity and community structure of postdisturbance forest soils as determined by sole-carbon-source utilization patterns. Microb Ecol 34(2):125–130
Stouvenakers G, Dapprich P, Massart S, Jijakli MH (2019) Plant pathogens and control strategies in aquaponics. In: Goddek S, Joyce A, Kotzen B, Burnell G (eds) Aquaponics food production systems. Springer, New York
Suleiman A, Lourenço KS, Pitombo LM, Mendes LW, Roesch L, Pijl A, Carmo JB, Cantarella H, Kuramae EE (2018) Recycling organic residues in agriculture impacts soil-borne microbial community structure, function and N2O emissions. Sci Tot Environ 631:1089–1099
Tresse O, Mounien F, Lévesque MJ, Guiot S (2005) Comparison of the microbial population dynamics and phylogenetic characterization of a CANOXIS reactor and a UASB reactor degrading trichloroethene. J Appl Microbiol 98(2):440–449
Vijayan K, Ravikumar G, Tikader A (2018) Mulberry (Morus spp.) breeding for higher fruit production. In: Al-Khayri J, Jain S, Johnson D (eds) Advances in plant breeding strategies: fruits. Springer, New York. https://doi.org/https://doi.org/10.1007/978-3-319-91944-7_3
Vittaladevaram V (2017) Fermentative production of microbial enzymes and their applications: present status and future prospects. J Appl Biol Biotechnol 5(4):90–94. https://doi.org/10.7324/JABB.2017.50414
Wang GF, Xie GL, Zhu B, Huang JS, Liu B, Kawicha P, Benyon L, Duan YP (2010a) Identification and characterization of the Enterobacter complex causing mulberry (Morus alba) wilt disease in China. Eur J Plant Pathol 126(4):465–478
Wang G, Xie G, Zhu B, Huang J, Liu B, Kawicha P, Benyon L, Duan Y (2010b) Identification and characterization of the Enterobacter complex causing mulberry (Morus alba) wilt disease in China. Eur J Plant Pathol 126:465–478. https://doi.org/10.1007/s10658-009-9552-x
Wang HY, Gao YB, Fan QW, Xu Y (2011) Characterization and comparison of microbial community of different typical Chinese liquor Daqus by PCR–DGGE. Lett Appl Microbiol 53(2):134–140
Were HK, Kabira JN, Kinyua ZM, Olubayo FM, Karinga JK, Aura J, Lees AK, Cowan GH, Torrance L (2013) Occurrence and distribution of potato pests and diseases in Kenya. Potato Res 56:325–342. https://doi.org/10.1007/s11540-013-9246-9
Xiao Y, Liu X, Meng D, Tao J, Gu Y, Yin H, Li J (2018) The role of soil bacterial community during winter fallow period in the incidence of tobacco bacterial wilt disease. Appl Microbiol Biotechnol 102(5):2399–2412
Yamazaki H, Kikuchi S, Hoshina T, Kimura T (2000) Effect of calcium concentration in nutrient solution on development of bacterial wilt and population of its pathogen Ralstonia solanacearum in grafted tomato seedlings. Soil Sci Plant Nutr 46(2):535–539
Yu C, Hu X, Deng W, Li Y, Han G, Ye C (2016) Soil fungal community comparison of different mulberry genotypes and the relationship with mulberry fruit sclerotiniosis. Sci Rep 6:28365
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26(9):1101–1108
Zhao Q, Wu J, Zhang L, Xu L, Yan C, Gong Z (2018) Identification and characterization of Cucurbita gummy stem blight fungi in Northeast China. J Phytopathol 166:305–313. https://doi.org/10.1111/jph.12688
Acknowledgements
The project was financially supported by the National Natural Science Foundation of China (No. 31770660 and 31300518) and the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-22).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, Z., Guo, Y., Yu, C. et al. The dynamics in rhizosphere microbial communities under bacterial wilt resistance by mulberry genotypes. Arch Microbiol 203, 1107–1121 (2021). https://doi.org/10.1007/s00203-020-02098-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02098-1