Abstract
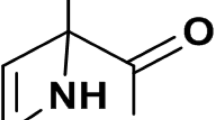
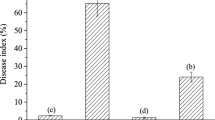
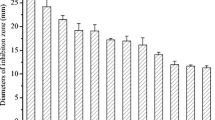
A novel antifungalmycin N2 (3-methyl-3,5-amino-4-vinyl-2-pyrone, C6H7O2N) was previously discovered from Streptomyces sp. N2, which exerted a broad-spectrum antagonistic activity against phytopathogenic fungi. To provide comprehensive insights into the antagonistic mechanisms and biocontrol efficacy of antifungalmycin N2, the present work investigated the physiological responses of Rhizoctonia solani under interaction with antifungalmycin N2. First, the mycelial growth of R. solani was significantly inhibited by antifungalmycin N2 during liquid shake-flask culture. Morphological observations showed that the morphogenesis of R. solani was influenced by antifungalmycin N2, in which the hyphae became severely shriveled and flattened, irregularly folded and branched. Additionally, an obvious accumulation of reactive oxygen species (ROS) was detected in R. solani hyphae, indicating oxidative stress induced by antifungalmycin N2. Further results showed that chitinase activity and its hydrolytic N-acetylglucosamine were significantly accelerated by antifungalmycin N2, demonstrating the cell wall of R. solani was damaged. Interestingly, the enzymatic antioxidant activities of R. solani were significantly induced in response to a relatively low concentration of antifungalmycin N2 (1.44–5.77 µg/mL). However, all antioxidant enzymes became highly inactive when the antifungalmycin N2 was increased to 11.53 µg/mL, suggesting that the enzymatic antioxidant system in R. solani was probably collapsed by the oxidative stress beyond its acceptance scope. In conclusion, antifungalmycin N2 exerted its antagonistic activity by inducing both cell wall degradation and oxidative stress in R. solani, thus leading to fungal morphogenesis and autolysis. Meanwhile, R. solani could induce and activate its antioxidant enzymes as a defence response to the oxidative stress caused by antifungalmycin N2.




Similar content being viewed by others
References
Aguirre J, Rios-Momberg M, Hewitt D, Hansberg W (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol 13:111–118
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Bio 55:373–399
Basu A, Chowdhury S, Ray Chaudhuri T, Kindu S (2016) Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant Pathol 8:1333–1346
Belozerskaya TA, Gessler NN (2007) Reactive oxygen species and the strategy of antioxidant defense in fungi: a review. Appl Biochem Microbiol 43:506–515
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. Bioessays 28:799–808
Chen YF, Zhou DB, Qi DF, Gao ZF, Xie JH, Luo YP (2017) Growth promotion and disease suppression ability of a Streptomyces sp. CB-75 from banana rhizosphere soil. Front Microbiol 8:2704
Crowe JD, Olsson S (2001) Induction of laccase activity in Rhizoctonia solani by antagonistic Pseudomonas fluorescens strains and a range of chemical treatments. Appl Environ Microbiol 67:2088–2094
Duffy B, Schouten A, Raaijmakers JM (2003) Pathogen self-defense: mechanisms to counteract microbial antagonism. Annu Rev Phytopathol 41:501–538
Feng SJ, Shu CW, Wang CJZ, Jiang SF, Zhou EX (2017) Survival of Rhizoctonia solani AG-1 IA, the causal agent of rice sheath blight, under different environmental conditions. J Phytopathol 165:44–52
Fuentefria AM, Pippi B, Dalla Lana DF, De Andrade SF (2017) Antifungals discovery: an insight into new strategies to combat antifungal resistance. Lett Appl Microbiol 66:2–13
Ghosh PG, Roy A, Hess D, Ghosh A, Das S (2015) Deciphering the mode of action of a mutant Allium sativum Leaf Agglutinin (mASAL), a potent antifungal protein on Rhizoctonia solani. BMC Microbiol 15:237
Gkarmiri K, Finlay RD, Alstörm S, Thomas E, Cubeta MA, Högberg N (2015) Transcriptomic changes in the plant pathogenic fungus Rhizoctonia solani AG-3 in response to the antagonistic bacteria Serratia proteamaculans and Serratia plymuthica. BMC Genom 16:630
Gohel V, Singh A, Vimal M, Ashwini P, Chatpar HS (2006) Bioprospecting and antifungal potential of chitynolitic microorganisms. Afr J Biotechnol 5:54–72
Hahn M (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Bio 7:133–141
Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp.. Phytopathology 96:190–194
Heller J, Tudzynski P (2011) Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annu Rev Phytopathol 49:369–390
Hoster F, Schmitz JE, Daniel R (2005) Enrichment of chitinolytic microorganisms: isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain. Appl Microbiol Biotechnol 66:434–442
Labuschagne CF, Brenkman AB (2013) Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res Rev 12:918–930
Lehtonen MJ, Wilson PS, Ahvenniemi P, Valkonen JPT (2009) Formation of canker lesions on stems and black scurf on tubers in experimentally inoculated potato plants by isolates of AG2-1, AG3 and AG5 of Rhizoctonia solani: a pilot study and literature review. Agric Food Sci 18:223–233
Lupetti A, Danesi R, Campa M, Tacca MD, Kelly S (2002) Molecular basis of resistance to azole antifungals. Trends Mol Med 8:76–81
Lushchak VI (2011) Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Phys C 153:175–190
Mazzola M, Freilich S (2016) Prospects for biological soil-borne disease control: application of indigenous versus synthetic microbiomes. Phytopathology 107:256–263
McSpadden Gardener BB, Driks A (2004) Overview of the nature and application of biocontrol microbes: Bacillus spp.. Phytopathology 94:1244
Narayanasamy P (2013) Mechanisms of action of fungal biological control agents. In: Biological management of diseases of crops. progress in biological control, vol 14. Springer, Amsterdam, pp 99–200
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Sahai AS, Manocha MS (1993) Chitinases of fungi and plants: their involvement in a morphogenesis and host/parasite interaction. FEMS Microbiol Rev 11:317–338
Tahvonen RT (2010) Microbial control of plant diseases with Streptomyces spp. 1. Eppo Bull 18:55–59
Tiwari IM, Jesuraj A, Kamboj R, Devanna BN, Botella JR, Sharma TR (2017) Host delivered RNAi, an efficient approach to increase rice resistance to sheath blight pathogen (Rhizoctonia solani). Sci Rep 7:7521
Ubhayasekera W, Karlsson M (2012) Bacterial and fungal chitinase ChiJ orthologs evolve under different selective constraints following horizontal transfer. BMC Res Notes 5:581
Wahleithner JA, Xu F, Brown KM, Brown SH, Golightly EJ, Halkier T, Kauppinen S, Pederson A, Schneider P (1996) The identification and characterization of four laccases from the plant-pathogenic fungus Rhizoctonia solani. Curr Genet 29:395–403
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256
Wu ZM, Yang Y, Li KT (2019) Antagonistic activity of a novel antifungalmycin N2 from Streptomyces sp. N2 and its biocontrol efficacy against Rhizoctonia solani. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnz018
Xu B, Chen W, Wu ZM, Long Y, Li KT (2015) A novel and effective Streptomyces sp. N2 against various phytopathogenic Fungi. Appl Biochem Biotechnol 177:1338–1347
Yellareddygari SKR, Reddy MS, Kloepper JW, Lawrence KS, Fadamiro H (2014) Rice sheath blight: a review of disease and pathogen management approaches. J Plant Pathol Microbiol 5:4
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 31760546), and the Training Program for Young Scientists of Jiangxi Provincial Department of Science and Technology (20142BCB23025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Wu, Zm. & Li, Kt. The peculiar physiological responses of Rhizoctonia solani under the antagonistic interaction coupled by a novel antifungalmycin N2 from Streptomyces sp. N2. Arch Microbiol 201, 787–794 (2019). https://doi.org/10.1007/s00203-019-01645-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01645-9