Abstract
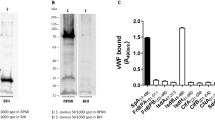
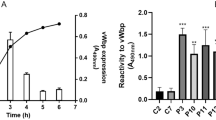
Many bacteria exploit host proteins for their colonization. Vitronectin (Vn), present in the blood and extracellular matrix, is one such protein that acts as a bridge between the bacteria and the host tissues leading to infection. In this study, Vn binding protein of Staphylococcus aureus (COL strain) (SaVnBP) has been characterized as autolysin(s) based on mass spectrometry data and the ability of these proteins to degrade S. aureus substratum. Deletion of the heparin-binding domain (residues 341–380) from the Vn did not affect its ability to interact with SaVnBP. Similarly, change of R to A or D to A in the second arginine–glycine–aspartic (RGD2) motif of Vn had no negative effect on protein–protein interaction. These results imply that the primary heparin-binding site and the second RGD motif of caprine Vn may not be involved in the initial step of S. aureus colonization.






Similar content being viewed by others
Abbreviations
- Vn:
-
Vitronectin
- HBD:
-
Heparin-binding domain
- RGD:
-
Arg–gly–asp
- SaVnBP:
-
S. aureus Vn binding protein
References
Chhatwal GS, Preissner KT, Muller-Berghaus G, Blobel H (1987) Specific binding of the human S protein (vitronectin) to Streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun 55:1878–1883
Duensing TD, Wing JS, Van-Putten JPM (1999) Sulfated polysaccharide directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis. Infect Immun 67:4463–4468
Emini EA, Hughes JV, Perlow DS, Boger J (1985) Induction of hepatitis A virus neutralizing antibody by a virus-specific synthetic peptide. J Virol 55:836–839
Foster TJ (2004) The Staphylococcus aureus “superbug”. J Clin Invest 114:1693–1696
Francois PP, Preissner KT, Herrmann M, Haugland RP, Vaudaux P, Lew DP, Krause KH (1999) Vitronectin interaction with glycosaminoglycans. Kinetics, structural determinants, and role in binding to endothelial cells. J Biol Chem 274:37611–37619
Heilmann C, Hussain M, Peters G, Gotz F (1997) Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol 24:1013–1024
Heilmann C, Thumm G, Chhatwal GS, Hartleib J, Uekotter A, Peters G (2003) Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 149:2769–2778
Heilmann C, Hartleib J, Hussain MS, Peters G (2005) The multifunctional Staphylococcus aureus autolysin aaa mediates adherence to immobilized fibrinogen and fibronectin. Infect Immun 73:4793–4802
Hirschhausen N, Schlesier T, Peters G, Heilmann C (2012) Characterization of the modular design of the autolysin/adhesin aaa from Staphylococcus aureus. PLoS One 77:e40353. https://doi.org/10.1371/journal.pone.0040353
Hussain M, Becker K, Eiff C von, Schrenzel J, Peters G, Herrmann M (2001) Identification and characterization of a novel 38.5 kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J Bacteriol 183:6778–6786
Kohler TP, Gisch N, Binsker U, Schlag M, Darm K, Völker U, Zähringer U, Hammerschmidt S (2013) Repeating structures of the major staphylococcal autolysin are essential for the interaction with human thrombospondin-1 and vitronectin. J Biol Chem 289:4070–4082
Komatsuzawa H, Sugai M, Nakashima S, Yamada S, Matsumoto A, Oshida T, Suginaka H (1997) Subcellular localization of the major autolysin, ATL and its processed proteins in Staphylococcus aureus. Microbiol Immunol 41:469–479
Leavesley DI, Kashyap AS, Croll T, Sivaramakrishnan M, Shokoohmand A, Hollier BG, Upton Z (2013) Vitronectin—master controller or micromanager? IUBMB Life https://doi.org/10.1002/iub.1203
Li D, Kirill G, Kjeld S, Puzas E, O’Keefe RJ, Awad H, Drissi H, Schwartz EM (2008) Quantitative mouse model of implant associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthopaedic Res 26:96–105
Liang OD, Flock JI, Wadstrom T (1995) Isolation and characterisation of a vitronectin-binding surface protein from Staphylococcus aureus. Biochim Biophys Acta 1250:110–116
Liang OD, Preissner KT, Chhatwal GS (1997) The hemopexin type repeats of human vitronectin are recognized by Streptococcus pyogenes. Biochem Biophys Res Commun 234:445–449
Mahawar M, Joshi P (2007) Goat vitronectin: characterization and binding to Staphylococcus aureus. Comp Biochem Physiol 149:410–418
Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A (1995) A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci USA 92:285–289
Porayath C, Suresh MK, Biswas R, Nair BG, Mishra N, Pal S (2018) Autolysin mediated adherence of Staphylococcus aureus with fibronectin, gelatin and heparin. Int J Biol Macromol 110:170–184
Qi D, Scholthof KB (2008) A one step PCR-based method for rapid and efficient site-directed fragment deletion, insertion and substitution mutagenesis. J Virol Methods 149:85–90
Rao TP, Prasanth TL, Murugavel S, Devi K, Joshi P (2017) Identification of second arginine–glycine–aspartic acid motif of caprine vitronectin as the complement C9 binding site and its implication in bacterial infection. Microbiol Immunol. https://doi.org/10.1111/1348-0421.12468
Sahoo S, Murugavel S, Devi K, Vedamurthy GV, Gupta SC, Singh BP, Joshi P (2013) Glyceraldehyde-3-phosphate dehydrogenase of the parasitic nematode Haemonchus contortus binds to complement C9 and inhibits its activity. Parasite Immunol 35:457–467
Schvartz I, Seger D, Shaltiel S (1999) Vitronectin. Int J Biochem Cell Biol 31:539–544
Shibata Y, Kawada M, Nakano Y, Toyoshima K, Yamashita Y (2005) Identification and characterization of an autolysin encoding gene of Streptococcus mutans. Infec Immun 73:3512–3520
Singh B, Su YC, Riesbeck K (2010) Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Mol Microbiol 78:545–560
Singh B, Su YC, Riesbeck K (2011) Vitronectin in host pathogen interactions and antimicrobial therapeutic applications. Cent Eur J Biol 6:973–980
Todor K (2004) Todar’s online textbook of bacteriology. Department of Bacteriology, University of Wisconsin-Madison, USA
Varrone JJ, Li D, Daiss JL, Schwarz EM (2011) Antiglucosaminadase Monoclonal antibodies as a passive immunization for methicillin—resistant Staphylococcus aureus (MRSA) orthopaedic infections. Bonekey Osteovis 1:187–194
Acknowledgements
We thank Director, IVRI for providing the facilities and our colleagues for encouragement. PJ was supported by grants from the Indian Council of Medical Research, New Delhi and the Department of Biotechnology, Government of India. HP and LP were supported by IVRI and MS by ICMR, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Communicated by Yusuf Akhter.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pathak, H., Sokkalingam, M., Prasanth, L. et al. Staphylococcus aureus autolysins interact with caprine vitronectin without involving the heparin binding domain and the second arginine–glycine–aspartic acid motif of the host protein. Arch Microbiol 201, 639–647 (2019). https://doi.org/10.1007/s00203-019-01624-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01624-0