Abstract
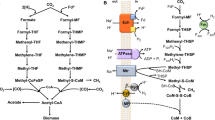
The anaerobic, acetogenic bacterium Acetobacterium woodii grows on hydrogen and carbon dioxide and uses the Wood–Ljungdahl pathway to fix carbon but also to synthesize ATP. The free energy change of acetogenesis from H2 + CO2 allows for synthesis of only a fraction of an ATP under environmental conditions, and A. woodii is clearly a paradigm for microbial life under extreme energy limitation. However, it was unknown how much energy is required to make ATP under these conditions. In the present study, we determined the phosphorylation potential in cells metabolizing three different acetogenic substrates. It accounts to 37.9 ± 1.3 kJ/mol ATP during acetogenesis from fructose, 32.1 ± 0.3 kJ/mol ATP during acetogenesis from H2 + CO2 and 30.2 ± 0.9 kJ/mol ATP during acetogenesis from CO, the lowest phosphorylation potential ever described. The physiological consequences in terms of energy conservation under extreme energy limitation are discussed.





Similar content being viewed by others
References
Bar-Even A (2013) Does acetogenesis really require especially low reduction potential? Biochim Biophys Acta 1827:395–400
Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD (2009) Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol 5:593–599
Berg JM, Tymoczko JL, Stryer L (2010) Biochemie, 6th edn. Spektrum Akademischer Verlag, München
Biegel E, Müller V (2010) Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase. Proc Natl Acad Sci USA 107:18138–18142
Blaut M, Gottschalk G (1984) Protonmotive force-driven synthesis of ATP during methane formation from molecular hydrogen and formaldehyde or carbon dioxide in Methanosarcina barkeri. FEMS Microbiol Lett 24:103–107
Boenigk R, Dürre P, Gottschalk G (1989) Carrier-mediated acetate transport in Acetobacterium woodii. Arch Microbiol 152:589–593
Borek E, Ryan A, Rockenbach J (1955) Nucleic acid metabolism in relation to the lysogenic phenomenon. J Bacteriol 69:460–467
Buckel W, Thauer RK (2013) Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim Biophys Acta 1827:94–113
Chapman AG, Fall L, Atkinson DE (1971) Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol 108:1072–1086
Giersch C, Robinson SP (1987) Regulation of photosynthetic carbon metabolism during phosphate limitation of photosynthesis in isolated spinach chloroplasts. Photosynth Res 14:211–227
Guffanti AA, Bornstein RF, Krulwich TA (1981) Oxidative phosphorylation by membrane vesicles from Bacillus alcalophilus. Biochim Biophys Acta 635:619–630
Hansen B, Bokranz M, Schönheit P, Kröger A (1988) ATP formation coupled to caffeate reduction by H2 in Acetobacterium woodii NZva16. Arch Microbiol 150:447–451
Harold FM, Spitz E (1975) Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol 122:266–277
Heinonen JE, Lahti RJ (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317
Heise R (1992) Energiekonservierung in Acetobacterium woodii: Aufbau eines elektrochemischen Na+-Gradienten und Nutzung durch eine ATP-Synthase mit Na+ als Kopplungsion. Dissertation, Georg-August-Universität zu Göttingen
Heise R, Müller V, Gottschalk G (1989) Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium woodii. J Bacteriol 171:5473–5478
Heise R, Müller V, Gottschalk G (1993) Acetogenesis and ATP synthesis in Acetobacterium woodii are coupled via a transmembrane primary sodium ion gradient. FEMS Microbiol Lett 112:261–268
Hess V, Schuchmann K, Müller V (2013) The ferredoxin:NAD+ oxidoreductase (Rnf) from the acetogen Acetobacterium woodii requires Na+ and is reversibly coupled to the membrane potential. J Biol Chem 288:31496–31502
Hess V, Poehlein A, Weghoff MC, Daniel R, Müller V (2014) A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genom 15:1139
Imkamp F, Müller V (2002) Chemiosmotic energy conservation with Na+ as the coupling ion during hydrogen-dependent caffeate reduction by Acetobacterium woodii. J Bacteriol 184:1947–1951
Jetten MSM, Stams AJM, Zehnder AJB (1991) Adenine nucleotide content and energy charge of Methanothrix soehngenii during acetate degradation. FEMS Microbiol Lett 84:313–317
Kashket ER (1982) Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry 21:5534–5538
Kimmich GA, Randles J, Brand JS (1975) Assay of picomole amounts of ATP, ADP and AMP using the luciferase enzyme system. Anal Biochem 69:187–206
Maloney PC (1983) Relationship between phosphorylation potential and electrochemical H+ gradient during glycolysis in Streptococcus lactis. J Bacteriol 153:1461–1470
Martin WF, Sousa FL, Lane N (2014) Evolution. Energy at life’s origin. Science 344:1092–1093
Matthies D, Zhou W, Klyszejko AL, Anselmi C, Yildiz Ö, Brandt K, Müller V, Faraldo-Gomez JD, Meier T (2014) High-resolution structure and mechanism of Na+-coupled F/V-hybrid ATP synthase rotor ring. Nat Commun 5:5286
Metelkin E, Demin O, Kovács Z, Chinopoulos C (2009) Modeling of ATP–ADP steady-state exchange rate mediated by the adenine nucleotide translocase in isolated mitochondria. FEBS J 276:6942–6955
Müller V (2003) Energy conservation in acetogenic bacteria. Appl Environ Microbiol 69:6345–6353
Müller V, Blaut M, Gottschalk G (1993) Bioenergetics of methanogenesis. In: Ferry JG (ed) Methanogenesis. Chapman & Hall, New York, pp 360–406
Müller-Esterl W (2004) Biochemie, 1st edn. Spektrum Akademischer Verlag, München
Poehlein A, Schmidt S, Kaster A-K, Goenrich M, Vollmers J, Thürmer A, Bertsch J, Schuchmann K, Voigt B, Hecker M, Daniel R, Thauer RK, Gottschalk G, Müller V (2012) An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7:e33439
Ragsdale SW (2008) Enzymology of the Wood–Ljungdahl pathway of acetogenesis. Ann N Y Acad Sci 1125:129–136
Schmidt K, Liaanen-Jensen S, Schlegel HG (1963) Die Carotinoide der Thiorodaceae. Arch Mikrobiol 46:117–126
Schuchmann K, Müller V (2014) Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12:809–821
Silverstein TP (2014) An exploration of how the thermodynamic efficiency of bioenergetic membrane systems varies with c-subunit stoichiometry of F1F0 ATP synthases. J Bioenerg Biomembr 46:229–241
Slater EC, Rosing J, Mol A (1973) The phosphorylation potential generated by respiring mitochondria. Biochim Biophys Acta 292:534–553
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Wallace PG, Pedler SM, Wallace JC, Berry MN (1994) A method for the determination of the cellular phosphorylation potential and glycolytic intermediates in yeast. Anal Biochem 222:404–408
Wood HG, Ljungdahl LG (1991) Autotrophic character of the acetogenic bacteria. In: Shively JM, Barton LL (eds) Variations in autotrophic life. Academic press, San Diego, pp 201–250
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 807). We thank K. Schuchmann for critical reading and discussion of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Rother.
Rights and permissions
About this article
Cite this article
Spahn, S., Brandt, K. & Müller, V. A low phosphorylation potential in the acetogen Acetobacterium woodii reflects its lifestyle at the thermodynamic edge of life. Arch Microbiol 197, 745–751 (2015). https://doi.org/10.1007/s00203-015-1107-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-015-1107-2