Abstract
Uridine triphosphate (UTP)-glucose-1-phosphate uridylyltransferase (GalU; EC 2.7.7.9) is an enzyme that catalyzes the formation of uridine diphosphate (UDP)-glucose from UTP and glucose-1-phosphate. GalU is involved in virulence in a number of animal-pathogenic bacteria since its product, UDP-glucose, is indispensable for the biosynthesis of virulence factors such as lipopolysaccharide and exopolysaccharide. However, its function in Xanthomonas campestris pv. campestris, the phytopathogen that causes black rot in cruciferous plants, is unclear. Here, we characterized a galU mutant of X. campestris pv. campestris and showed that the X. campestris pv. campestris galU mutant resulted in a reduction in virulence on the host cabbage. We also demonstrated that galU is involved in bacterial attachment, cell motility, and polysaccharide synthesis. Furthermore, the galU mutant showed increased sensitivity to various stress conditions including copper sulfate, hydrogen peroxide, and sodium dodecyl sulfate. In addition, mutation of galU impairs the expression of the flagellin gene fliC as well as the attachment-related genes xadA, fhaC, and yapH. In conclusion, our results indicate involvement of galU in the virulence factor production and pathogenicity in X. campestris pv. campestris, and a role for galU in stress tolerance of this crucifer pathogen.




Similar content being viewed by others
References
Aragao D, Fialho AM, Marques AR, Mitchell EP, Sa-Correia I, Frazao C (2007) The complex of Sphingomonas elodea ATCC 31461 glucose-1-phosphate uridylyltransferase with glucose-1-phosphate reveals a novel quaternary structure, unique among nucleoside diphosphate-sugar pyrophosphorylase members. J Bacteriol 189:4520–4528. doi:10.1128/JB.00277-07
Bogdanove AJ et al (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol 193:5450–5464. doi:10.1128/JB.05262-11
Bosco MB, Machtey M, Iglesias AA, Aleanzi M (2009) UDPglucose pyrophosphorylase from Xanthomonas spp. Characterization of the enzyme kinetics, structure and inactivation related to oligomeric dissociation. Biochimie 91:204–213. doi:10.1016/j.biochi.2008.09.001
Buttner D, Bonas U (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34:107–133. doi:10.1111/j.1574-6976.2009.00192.x
Chan JW, Goodwin PH (1999) The molecular genetics of virulence of Xanthomonas campestris. Biotechnol Adv 17:489–508
Chang HY, Lee JH, Deng WL, Fu TF, Peng HL (1996) Virulence and outer membrane properties of a galU mutant of Klebsiella pneumoniae CG43. Microb Pathog 20:255–261. doi:10.1006/mpat.1996.0024
da Silva AC et al (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459–463
Darsonval A, Darrasse A, Durand K, Bureau C, Cesbron S, Jacques MA (2009) Adhesion and fitness in the bean phyllosphere and transmission to seed of Xanthomonas fuscans subsp. fuscans. Mol Plant Microbe Interact 22:747–757. doi:10.1094/MPMI-22-6-0747
Das A, Rangaraj N, Sonti RV (2009) Multiple adhesin-like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol Plant Microbe Interact 22:73–85. doi:10.1094/MPMI-22-1-0073
Deng WL et al (2010) Effects of galU mutation on Pseudomonas syringae-plant interactions. Mol Plant Microbe Interact 23:1184–1196. doi:10.1094/MPMI-23-9-1184
Dow JM, Daniels MJ (1994) Pathogenicity determinants and global regulation of pathogenicity of Xanthomonas campestris pv. campestris. Curr Top Microbiol Immunol 192:29–41
Dow JM, Osbourn AE, Wilson TJ, Daniels MJ (1995) A locus determining pathogenicity of Xanthomonas campestris is involved in lipopolysaccharide biosynthesis. Mol Plant Microbe Interact 8:768–777
Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc Natl Acad Sci U S A 100:10995–11000. doi:10.1073/pnas.1833360100
Fu JF, Tseng YH (1990) Construction of lactose-utilizing Xanthomonas campestris and production of xanthan gum from whey. Appl Environ Microbiol 56:919–923
Genevaux P, Bauda P, DuBow MS, Oudega B (1999) Identification of Tn10 insertions in the rfaG, rfaP, and galU genes involved in lipopolysaccharide core biosynthesis that affect Escherichia coli adhesion. Arch Microbiol 172:1–8
Gottig N, Garavaglia BS, Garofalo CG, Orellano EG, Ottado J (2009) A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS One 4:e4358. doi:10.1371/journal.pone.0004358
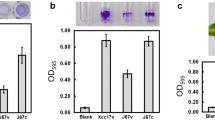
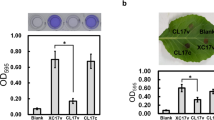
Guo Y, Sagaram US, Kim JS, Wang N (2010) Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in planta of Xanthomonas citri subsp. citri. Appl Environ Microbiol 76:2234–2242. doi:10.1128/AEM.02897-09
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hsiao YM, Liao HY, Lee MC, Yang TC, Tseng YH (2005) Clp upregulates transcription of engA gene encoding a virulence factor in Xanthomonas campestris by direct binding to the upstream tandem Clp sites. FEBS Lett 579:3525–3533
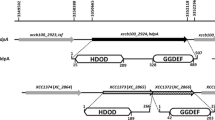
Hsiao YM, Liu YF, Fang MC, Song WL (2011) XCC2731, a GGDEF domain protein in Xanthomonas campestris, is involved in bacterial attachment and is positively regulated by Clp. Microbiol Res 166:548–565. doi:10.1016/j.micres.2010.11.003
Hsiao YM, Song WL, Liao CT, Lin IH, Pan MY, Lin CF (2012) Transcriptional analysis and functional characterization of XCC1294 gene encoding a GGDEF domain protein in Xanthomonas campestris pv. campestris. Arch Microbiol 194:293–304. doi:10.1007/s00203-011-0760-3
Jalan N et al (2011) Comparative genomic analysis of Xanthomonas axonopodis pv. citrumelo F1, which causes citrus bacterial spot disease, and related strains provides insights into virulence and host specificity. J Bacteriol 193:6342–6357. doi:10.1128/JB.05777-11
Jiang SS, Lin TY, Wang WB, Liu MC, Hsueh PR, Liaw SJ (2010) Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrob Agents Chemother 54:2000–2009. doi:10.1128/AAC.01384-09
Keen NT, Tamaki S, Kobayashi D, Trollinger D (1988) Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191–197
Kim H et al (2010) Structural basis for the reaction mechanism of UDP-glucose pyrophosphorylase. Mol Cells 29:397–405. doi:10.1007/s10059-010-0047-6
Komeda Y, Icho T, Iino T (1977) Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol 129:908–915
Lee TC et al (2001) The early stages of filamentous phage ϕLf infection require the host transcription factor, Clp. J Mol Microbiol Biotechnol 3:471–481
Lee MC, Weng SF, Tseng YH (2003) Flagellin gene fliC of Xanthomonas campestris is upregulated by transcription factor Clp. Biochem Biophys Res Commun 307:647–652
Lee BM et al (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucleic Acids Res 33:577–586
Lesse AJ, Campagnari AA, Bittner WE, Apicella MA (1990) Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods 126:109–117
Liu YF et al (2013) GsmR, a response regulator with an HD-related output domain in Xanthomonas campestris, is positively controlled by Clp and is involved in the expression of genes responsible for flagellum synthesis. FEBS J 280:199–213. doi:10.1111/febs.12061
McCarthy Y et al (2008) The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol Plant Pathol 9:819–824. doi:10.1111/j.1364-3703.2008.00495.x
Miller JH (1972) Experiments in molecular genetics. Cold Spring Habor Laboratory, Cold Spring Harbor, NY
Nesper J, Kapfhammer D, Klose KE, Merkert H, Reidl J (2000) Characterization of Vibrio cholerae O1 antigen as the bacteriophage K139 receptor and identification of IS1004 insertions aborting O1 antigen biosynthesis. J Bacteriol 182:5097–5104
Nesper J, Lauriano CM, Klose KE, Kapfhammer D, Kraiss A, Reidl J (2001) Characterization of Vibrio cholerae O1 El tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect Immun 69:435–445. doi:10.1128/IAI.69.1.435-445.2001
Pieretti I et al (2009) The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genomics 10:616
Priebe GP et al (2004) The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect Immun 72:4224–4232. doi:10.1128/IAI.72.7.4224-4232.2004
Qian W et al (2005) Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res 15:757–767
Rioux S et al (1999) Isolation and characterization of mini-Tn10 lipopolysaccharide mutants of Actinobacillus pleuropneumoniae serotype 1. Can J Microbiol 45:1017–1026
Ryan RP et al (2011) Pathogenomics of Xanthomonas: understanding bacterium-plant interactions. Nat Rev Microbiol 9:344–355. doi:10.1038/nrmicro2558
Salzberg SL et al (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics 9:204. doi:10.1186/1471-2164-9-204
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd. In. Cold Spring Habor Press, Cold Spring Harbor, NY
Thieme F et al (2005) Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol 187:7254–7266
Thoden JB, Holden HM (2007a) Active site geometry of glucose-1-phosphate uridylyltransferase. Protein Sci 16:1379–1388. doi:10.1110/ps.072864707
Thoden JB, Holden HM (2007b) The molecular architecture of glucose-1-phosphate uridylyltransferase. Protein Sci 16:432–440. doi:10.1110/ps.062626007
Vilches S et al (2007) Mesophilic Aeromonas UDP-glucose pyrophosphorylase (GalU) mutants show two types of lipopolysaccharide structures and reduced virulence. Microbiology 153:2393–2404. doi:10.1099/mic.0.2007/006437-0
Vorholter FJ, Niehaus K, Puhler A (2001) Lipopolysaccharide biosynthesis in Xanthomonas campestris pv. campestris: a cluster of 15 genes is involved in the biosynthesis of the LPS O-antigen and the LPS core. Mol Genet Genomics 266:79–95
Vorholter FJ et al (2008) The genome of Xanthomonas campestris pv. campestris B100 and its use for the reconstruction of metabolic pathways involved in xanthan biosynthesis. J Biotechnol 134:33–45
Wang TW, Tseng YH (1992) Electrotransformation of Xanthomonas campestris by RF DNA of filamentous phage ϕLf. Lett Appl Microbiol 14:65–68
Wei CL, Lin NT, Weng SF, Tseng YH (1996) The gene encoding UDP-glucose pyrophosphorylase is required for the synthesis of xanthan gum in Xanthomonas campestris. Biochem Biophys Res Commun 226:607–612. doi:10.1006/bbrc.1996.1403
Wengelnik K, Marie C, Russel M, Bonas U (1996) Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol 178:1061–1069
Williams PH (1980) Black rot: a continuing threat to world crucifers. Plant Dis 64:736–742
Yang BY, Tseng YH (1988) Production of exopolysaccharide and levels of protease and pectinase activity in pathogenic and non-pathogenic strains of Xanthomonas campestris pv. campestris. Bot Bull Acad Sin 29:93–99
Yen MR, Lin NT, Hung CH, Choy KT, Weng SF, Tseng YH (2002) oriC region and replication termination site, dif, of the Xanthomonas campestris pv. campestris 17 chromosome. Appl Environ Microbiol 68:2924–2933
Zou Y et al (2013) The role of galU and galE of Haemophilus parasuis SC096 in serum resistance and biofilm formation. Vet Microbiol 162:278–284. doi:10.1016/j.vetmic.2012.08.006
Acknowledgments
This work was supported by the National Science Council of Taiwan Grant No. NSC 101-2313-B-166-001-MY3 to Yi-Min Hsiao, and the Central Taiwan University of Science and Technology Grant No. CTU100-PC-010 to Chao-Tsai Liao.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, CT., Du, SC., Lo, HH. et al. The galU gene of Xanthomonas campestris pv. campestris is involved in bacterial attachment, cell motility, polysaccharide synthesis, virulence, and tolerance to various stresses. Arch Microbiol 196, 729–738 (2014). https://doi.org/10.1007/s00203-014-1012-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1012-0