Abstract
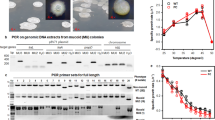
Legionella (Fluoribacter) dumoffii is a resident of various aquatic environments and occasionally causes pneumonia in humans. We found that L. dumoffii strain TEX-KL carries a 66-kb circular plasmid. As predicted by the presence of tra genes similar to those of other transferable plasmids, we showed that pLD-TEX-KL was actually capable of transferring itself to a plasmid-cured derivative of the original strain. Unexpectedly, this plasmid-free derivative turned out to be partially defective in terms of growth at temperatures 30°C or lower. Subsequent works revealed that the growth defect was attributable to the loss of the plasmid gene traA(Ti) homologous to the traA gene of Ti plasmid from Agrobacterium tumefaciens, and that the growth was restored by the introduction of the mobA/repB gene of plasmid pMMB207. Since the existence of a DNA nickase domain is the only feature common to the traA(Ti) and mobA/repB gene products, we hypothesized that this growth defect at low temperature is related to insufficient DNA transactions, which can somehow be alleviated by the nickase activity of those plasmid-encoded proteins. It was also noted that the above features of growth defect at low temperatures were seen in L. dumoffii cells parasitizing the amebic host Acanthamoeba culbertsoni.





Similar content being viewed by others
References
Baine WB (1985) Cytolytic and phospholipase C activity in Legionella species. J Gen Microbiol 131:1383–1391
Bozue JA, Johnson W (1996) Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun 64:668–673
Brenner DJ (1985) The new species of Legionella. Int J Syst Bacteriol 35:50–59
Cordes LG, Wilkinson HW, Gorman GW, Fikes BJ, Fraser DW (1979) Atypical Legionella-like organisms: fastidious water-associated bacteria pathogenic for man. Lancet 2:927–930
D’Amico S, Collins T, Marx JC, Feller G, Gerday C (2006) Psychrophilic microorganisms: challenges for life. EMBO Rep 7:385–389
de Lorenzo V, Herrero M, Jakubzik U, Timmis KN (1990) Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol 172:6568–6572
Fang GD, Yu VL, Vickers RM (1989) Disease due to the Legionellaceae (other than Legionella pneumophila). Historical, microbiological, clinical, and epidemiological review. Medicine (Baltimore) 68:116–132
Farrand SK, Hwang I, Cook DM (1996) The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J Bacteriol 178:4233–4247
Feeley JC et al (1979) Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol 10:437–441
Fields BS (1996) The molecular ecology of Legionellae. Trends Microbiol 4:286–290
Fields BS, Benson RF, Besser RE (2002) Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev 15:506–526
Goldstein E, Drlica K (1984) Regulation of bacterial DNA supercoiling: plasmid linking numbers vary with growth temperature. Proc Natl Acad Sci USA 81:4046–4050
Gorbalenya AE, Koonin EV (1993) Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol 3:419–429
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hirukawa Y, Nakato H, Izumi S, Tsuruhara T, Tomino S (1998) Structure and expression of a cyst specific protein of Acanthamoeba castellanii. Biochim Biophys Acta 1398:47–56
Jones PG, Krah R, Tafuri SR, Wolffe AP (1992) DNA gyrase, CS7.4, and the cold shock response in Escherichia coli. J Bacteriol 174:5798–5802
Kuroki H et al (2007) Legionella impletisoli sp. nov. and Legionella yabuuchiae sp. nov., isolated from soils contaminated with industrial wastes in Japan. Syst Appl Microbiol 30:273–279
Lewallen KR et al (1979) A newly identified bacterium phenotypically resembling, but genetically distinct from, Legionella pneumophila: an isolate in a case of pneumonia. Ann Intern Med 91:831–834
Maruta K, Miyamoto H, Hamada T, Ogawa M, Taniguchi H, Yoshida S (1998a) Entry and intracellular growth of Legionella dumoffii in alveolar epithelial cells. Am J Respir Crit Care Med 157:1967–1974
Maruta K, Ogawa M, Miyamoto H, Izu K, Yoshida S (1998b) Entry and intracellular localization of Legionella dumoffii in Vero cells. Microb Pathog 24:65–73
Moffat JF, Tompkins LS (1992) A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun 60:296–301
Morales VM, Backman A, Bagdasarian M (1991) A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39–47
Ohnishi H et al (2004) Legionella dumoffii DjlA, a member of the DnaJ family, is required for intracellular growth. Infect Immun 72:3592–3603
Park MY, Ko KS, Lee HK, Park MS, Kook YH (2003) Legionella busanensis sp. nov., isolated from cooling tower water in Korea. Int J Syst Evol Microbiol 53:77–80
Pearson WR, Wood T, Zhang Z, Miller W (1997) Comparison of DNA sequences with protein sequences. Genomics 46:24–36
Piao Z, Sze CC, Barysheva O, Iida K, Yoshida S (2006) Temperature-regulated formation of mycelial mat-like biofilms by Legionella pneumophila. Appl Environ Microbiol 72:1613–1622
Qin T et al (2007) Complete nucleotide sequence of pLD-TEX-KL, a 66-kb plasmid of Legionella dumoffii TEX-KL strain. Plasmid 58:261–268
Rawlings DE, Tietze E (2001) Comparative biology of IncQ and IncQ-like plasmids. Microbiol Mol Biol Rev 65:481–496 table of contents
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual, 3rd edn. Cold Spring Harvor Laboratry Press, New York
Soderberg MA, Cianciotto NP (2008) A Legionella pneumophila peptidyl-prolyl cis-trans isomerase present in culture supernatants is necessary for optimal growth at low temperatures. Appl Environ Microbiol 74:1634–1638
Soderberg MA, Rossier O, Cianciotto NP (2004) The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures. J Bacteriol 186:3712–3720
Wiater LA, Sadosky AB, Shuman HA (1994) Mutagenesis of Legionella pneumophila using Tn903 dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol Microbiol 11:641–653
Acknowledgments
We thank Hideko Kajiwara for technical assistance and Sharon Y. A. M. Villanueva for comments on the manuscript. We also thank Takayoshi Yamaguchi and Eiji Harada for useful suggestions. This work was supported by grant 14370094 from the Ministry of Education, Science, Culture and Sports of Japan and grant H14-047 from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sebastian Suerbaum.
Rights and permissions
About this article
Cite this article
Qin, T., Iida, Ki., Hirakawa, H. et al. Conjugative plasmid pLD-TEX-KL promotes growth of host bacterium Legionella dumoffii at low temperatures. Arch Microbiol 191, 543–551 (2009). https://doi.org/10.1007/s00203-009-0481-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-009-0481-z