Abstract
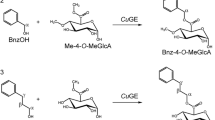
Glucuronoyl esterase is a novel carbohydrate esterase recently discovered in the cellulolytic system of the wood-rotting fungus Schizophyllum commune on the basis of its ability to hydrolyze methyl ester of 4-O-methyl-d-glucuronic acid. This substrate was not fully corresponding to the anticipated function of the enzyme to hydrolyze esters between xylan-bound 4-O-methyl-d-glucuronic acid and lignin alcohols occurring in plant cell walls. In this work we showed that the enzyme was capable of hydrolyzing two synthetic compounds that mimic the ester linkages described in lignin-carbohydrate complexes, esters of 4-O-methyl-d-glucuronic and d-glucuronic acid with 3-(4-methoxyphenyl)propyl alcohol. A comparison of kinetics of hydrolysis of methyl and 3-(4-methoxyphenyl)propyl esters indicated that the glucuronoyl esterase recognizes the uronic acid part of the substrates better than the alcohol type. The catalytic efficiency of the enzyme was much higher with the ester of 4-O-methyl-d-glucuronic acid than with that of d-glucuronic acid. Examination of the action of glucuronoyl esterase on a series of methyl esters of 4-O-methyl-d-glucopyranuronosyl residues α-1,2-linked to xylose and several xylooligosaccharides suggested that the rate of deesterification is independent of the character of the carbohydrate part glycosylated by the 4-O-methyl-d-glucuronic acid.


Similar content being viewed by others
References
Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes server at URL, http://www.afmb.cnrs-mrs.fr/CAZY
Crepin VF, Faulds CB, Connerton IF (2004) Functional calssification of microbial feruloyl esterases. Appl Microbiol Biotechnol 63:647–652
Das NN, Das SC, Mukherjee AK (1984a) On the ester linkage between lignin and 4-O-methyl-d-glucurono-d-xylan in jute fiber (Corchorus capsularis). Carbohydr Res 127:345–348
Das NN, Das SC, Sarkar AK, Mukherjee AK (1984b) Lignin–xylan ester linkage in mesta fiber (Hibiscus cannabinus). Carbohydr Res 129:197–207
Hestrin S (1949) The reaction of acetylcholine and other carboxylic acid derivates with hydroxylamine, and its analytical application. J Biol Chem 180:249–261
Hirsch J, Kováč P, Alföldi J, Mihálov V (1981) Synthesis of aldotriouronic acid derivatives related to (4-O-methylglucurono)xylans. Carbohydr Res 88:146–152
Hirsch J, Koóš M, Kováč P (1998) Improved synthesis of an aldobiouronic acid related to hardwood xylans, and preparation of a derivative thereof suitable for linking to proteins. Carbohydr Res 310:145–149
Hirsch J, Langer V, Koóš M (2005) Synthesis and molecular structure of methyl 4-O-methyl-α-d-glucopyranuronate. Molecules 10:251–258
Imamura T, Watanabe T, Kuwahara M, Koshijima T (1994) Ester linkages between lignin and glucuronic acid in lignin-carbohydrate complexes from Fagus crenata. Phytochem 37:1165–1173
Jeffries T (1990) Biodegradation of lignin-carbohydrate complexes. Biodegradation 1:163–176
Kováč P (1977) Synthesis and reactions of uronic acid derivatives. XVIII. The stepwise synthesis of a (4-O-methylglucurono)xylan type, branched aldotriouronic acid derivative. J Carbohydr Nucleos Nucleot 4:165–173
Kováč P, Palovčík R (1978) Synthesis and reactions of uronic acid derivates. XVII. Synthesis of methyl 2-O-(methyl 4-O-methyl-α- and β-d-glucopyranosyluronate)-β-d-xylopyranoside. Chem zvesti 32:501–513
Kováč P, Hirsch J, Kováčik V, Kočiš P (1980) The stepwise synthesis of an aldopentaouronic acid derivate related to branched (4-O-methylglucurono)xylans. Carbohydr Res 85:41–49
Poláková M, Joniak D, Ďuriš M (2000) Synthesis and acid-catalyzed hydrolysis of some 3-(4-methoxyphenyl)propyl glucuronates. Collect Czech Chem Commun 65:1609–1618
Špániková S, Biely P (2006) Glucuronoyl esterase – Novel carbohydrate esterase produced by Schizophyllum commune. FEBS Lett 580:4597–4601
Watanabe T, Koshijima T (1988) Evidence for an ester linkage between lignin and glucuronic acid in lignin-carbohydrate complexes by DDQ-oxidation. Agric Biol Chem 52:2953–2955
Acknowledgments
This work was supported by a Grant 2/6130/26 from the Slovak Grant Agency VEGA, a Grant from the Slovak Academy of Sciences to the Center of Excellence GLYCOBIOS and by a grant from the Slovak Research and Development Agency under the Contract No. APVV-51-003805.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Geoffrey Turner.
Rights and permissions
About this article
Cite this article
Špániková, S., Poláková, M., Joniak, D. et al. Synthetic esters recognized by glucuronoyl esterase from Schizophyllum commune . Arch Microbiol 188, 185–189 (2007). https://doi.org/10.1007/s00203-007-0241-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-007-0241-x