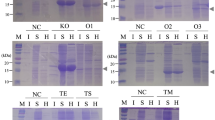
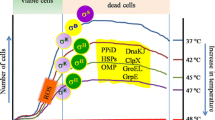
Abstract
The natural living style of Escherichia coli occurs in the gastrointestinal tract, where most of its existence is spent under anaerobic conditions and in stationary phase of growth. Here we report on the heat shock response of E. coli K-12 cells growing in the presence or absence of oxygen. An rpoH mutant (impaired in the synthesis of the σ32 transcriptional factor) exhibited an increased sensitivity to heat shock but only in the exponential phase of aerobic growth, suggesting that in anaerobic growth conditions, and in aerobic stationary phase, σ32-independent mechanisms are playing a prime role in protecting cells from heat stress. Our results demonstrated that σS is not involved in this protection system. Studies on the kinetics of synthesis of Heat shock proteins (Hsp) after an abrupt rise in temperature demonstrated that in the absence of oxygen, the synthesis of Hsp is triggered faster and is sustained for a longer period of time compared to aerobic growth conditions. Finally, the heated cells in the exponential phase of aerobic growth displayed a high concentration of oxidatively damaged proteins in the presence of 4 mM H2O2, in sharp contrast to cultures of stationary phase or anaerobic growth.






Similar content being viewed by others
References
Arsene F, Tomoyasu T, Bukau B (2000) The heat shock response of Escherichia coli. Intl J Food Microbiol 55:3–9
Bang IS, Frye JG, McClelland M, Velayudhan J, Fang FC (2005) Alternative sigma factor interactions in Salmonella: σE and σH promote antioxidant defences by enhancing σS levels. Mol Microbiol 56:811–823
Benov L, Fridovich I (1995) Superoxide dismutase protects against aerobic heat shock in Escherichia coli. J Bacteriol 177:3344–3346
Bromberg R, George SM, Peck MW (1998) Oxygen sensitivity of heated cells of Escherichia coli O157:H7. J Appl Microbiol 85:231–237
Camacho-Carranza R, Membrillo-Hernández J, Ramírez-Santos J, Castro-Dorantes J, Chagoya de Sánchez V, Gómez-Eichelmann MC (1995) Topoisomerase activity during the heat shock response in Escherichia coli K-12. J Bacteriol 177:3619–3622
Connoly L, Yura T, Gross CA (1999) Autoregulation of the heat shock response in prokaryotes. In: Bukau B (ed) Molecular Chaperones and folding catalysts. Regulation, cellular function and mechanism. Harwood Academic Publishers, Amsterdam, pp 13–33
Corona-Izquierdo FP, Membrillo-Hernández J (2002) A mutation in rpoS enhances biofilm formation in Escherichia coli during exponential phase of growth. FEMS Microbiol Lett 211:105–110
Delaney JM (1990) Requirement of the Escherichia coli dnaK gene for thermotolerance and protection against H2O2. Microbiology 136:2113–2118
Echave P, Esparza-Cerón MA, Cabiscol E, Tamarit J, Ros J, Membrillo-Hernández J, Lin ECC (2002) DnaK dependence of mutant ethanol oxidoreductases evolved for aerobic function and protective role of the chaperone against protein oxidative damage in Escherichia coli. Proc Natl Acad Sci USA 99:4626–4631
Fredriksson A, Ballesteros M, Dukan S, Nyström T (2006) Induction of the heat shock regulon in response to increased mistranslation requires oxidative modification of the malformed proteins. Mol Microbiol 59:350–359
Groat RG, Schultz JE, Zychlinsky E, Bockman A, Matin A (1986) Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol 168:486–493
Gruber TM, Gross CA (2003) Multiple σ subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol 57:441–466
Guerra E, Chye PP, Berardi E, Piper PW (2005) Hypoxia abolishes transience of the heat-shock response in the methylotrophic yeast Hansenula polymorpha. Microbiology 151:805–811
Guisbert E, Herman C, Lu CZ, Gross CA (2004) A chaperone network controls the heat shock response in E. coli. Genes Dev 18:2812–2821
Hengge-Aronis R (2000) The general stress response in Escherichia coli. In: Storz G, Hengge-Aronis (eds) Bacterial stress responses. ASM Press, Washington
Hengge-Aronis R (2002) Stationary phase gene regulation: what makes an Escherichia coli promoter sigmaS-selective? Curr Opin Microbiol 5:591–595
Jakob U, Muse W, Eser M, Bardwell JC (1999) Chaperone activity with a redox switch. Cell 96:341–352
Jenkins DE, Schultz JE, Matin A (1988) Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol 170:3910–3914
Jenkins DE, Auger EA, Matin A (1991) Role of RpoH, a heat shock regulator protein in Escherichia coli carbon starvation protein synthesis and survival. J Bacteriol 173:1992–1996
Jishage M, Ishihama A (1995) Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of σ70 and σ38. J Bacteriol 177:6832–6835
King T, Ferenci T (2005) Divergent roles of RpoS in Escherichia coli under aerobic and anaerobic conditions. FEMS Microbiol Lett 244:323–327
Kolter R, Siegele DA, Tormo A (1993) The stationary phase of the bacterial life cycle. Annu Rev Microbiol 47:855–874
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lange R, Hengge-Aronis R (1991) Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J Bacteriol 173:4474–4481
Lee-Rivera I, Gómez-Eichelmann MC (1994) Escherichia coli cells with mutations in the gene for adenylate cyclase (cya) exhibit a heat shock response. FEMS Microbiol Lett 121:35–38
Levine RL, Oliver CN, Fulks RM, Stadtman ER (1981) Turnover of bacterial glutamine synthetase: Oxidative inactivation precedes proteolysis. Proc Natl Acad Sci USA 78:2120–2124
Loewen PC, Hengge-Aronis R (1994) The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu Rev Microbiol 48:53–80
López-Sánchez F, Ramírez-Santos J, Gómez-Eichelmann MC (1997) In vivo effect of DNA relaxation on the transcription of gene rpoH in Escherichia coli. Biochim Biophys Acta 1353:79–83
Marqués S, Manzanera M, González-Pérez MM, Gallegos MT, Ramos JL (1999) The XylS-dependent Pm promoter is transcribed In vivo by RNA polymerase with σ32 or σ38 depending on the growth phase. Mol Microbiol 31:1105–1113
Membrillo-Hernández J, Echave P, Cabiscol E, Tamarit J, Ros J, Lin ECC (2000) Evolution of the adhE gene product of Escherichia coli from a functional reductase to a dehydrogenase. Genetic and biochemical studies of the mutant proteins. J Biol Chem 275:33869–33875
Membrillo-Hernández J, Lin ECC (1999) Regulation of expression of the adhE gene, encoding ethanol oxidoreductase in Escherichia coli: transcription from a downstream promoter and regulation by Fnr and RpoS. J Bacteriol 181:7571–7579
Miller JH (1972) In: Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Cold Spring Harbor, NY, USA
Mizushima T, Ohtsuka Y, Miki T, Sekimizu K (1994) Temperature shift-up leads to simultaneous and continuous plasmid DNA relaxation and induction of DnaK and GroEL proteins in anaerobically growing Escherichia coli cells. FEMS Microbiol Lett 121:333–336
Mogk A, Bukau B, Deuerling E (2001) Cellular functions of cytosolic Escherichia coli chaperones. In: Lund P (ed) Molecular Chaperones in the cell. Oxford University Press, Oxford
Morimoto RI, Kline MP, Blimston DN, Cotto JJ (1997) The heat–shock response: regulation and function of heat–shock proteins and molecular chaperones. Essays Biochem 32:17–29
Morita M, Kanemori M, Yanagi H, Yura T (1999a) Heat-induced synthesis of σ32 in Escherichia coli: structural and functional dissection of rpoH mRNA secondary structure. J Bacteriol 181:401–410
Morita M, Tanaka Y, Kodama TS, Kyogoku Y, Yanagi H, Yura T (1999b) Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev 13:655–665
Neidhardt FC, VanBogelen RA, Vaugh V (1984) The genetics and regulation of the heat-shock proteins. Annu Rev Genet 18:295–329
Nollen EA, Morimoto RI (2002) Chaperoning signalling pathways: Molecular chaperones as stress-sensing “heat shock” proteins. J Cell Sci 115:2809–2816
Nyström T (2005) Role of oxidative carbonylation in protein quality control and senescence. EMBO J 24:1311–1317
Reyes-Domínguez Y, Contreras-Ferrat G, Ramírez-Santos J, Membrillo-Hernández J, Gómez-Eichelmann MC (2003) Plasmid DNA supercoiling and gyrase activity in Escherichia coli wild-type and rpoS stationary-phase cells. J Bacteriol 185:1097–1100
Rockabrand D, Arthur T, Korinek G, Livers K, Blum P (1995) An essential role for the Escherichia coli DnaK protein in starvation-induced thermotolerance, H2O2 resistance, and reductive division. J Bacteriol 177:3695–3703
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, NY
Straus DB, Walter WA, Gross CA (1987) The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329:348–351
Tamarit J, Cabiscol E, Ros J (1998) Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem 273:3027–3032
VanBogelen RA, Acton MA, Neidhardt FC (1989) Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev 1:525–531
Williams MD, Ouyang TX, Flickinger MC (1994) Starvation-induced expression of SspA and SspB: the effects of a null mutation in sspA on Escherichia coli protein synthesis and survival during growth and prolonged starvation. Mol Microbiol 11:1029–1043
Winter J, Linke K, Jatzek A, Jakob U (2005) Severe oxidative stress causes inactivation of DnaK and activation of the redox-regulated chaperone Hsp33. Mol Cell 17:381–392
Acknowledgments
This work was supported by grants 42580-Q from CONACYT (Mexico), and 207703 from PAPIIT-UNAM (Mexico). Technical assistance from Miguel Páez is greatly appreciated. We thank members of the Membrillo-Hernández lab for helpful advises and Peter Lund for plasmid pAJW5. This paper is the manuscript #1 of the multi-group project on oxidative stress of the Biomedical Research Institute (UNAM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz-Acosta, A., Sandoval, M.L., Delgado-Olivares, L. et al. Effect of anaerobic and stationary phase growth conditions on the heat shock and oxidative stress responses in Escherichia coli K-12. Arch Microbiol 185, 429–438 (2006). https://doi.org/10.1007/s00203-006-0113-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0113-9