Abstract
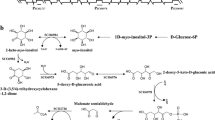
A gene encoding a γ-butyrolactone autoregulator receptor, which has a common activity as DNA-binding transcriptional repressors controlling secondary metabolism and/or morphological differentiation in Streptomyces, was cloned from a natamycin producer, Streptomyces natalensis. PCR using the primers designed for the two highly conserved regions of Streptomyces autoregulator receptors (BarA, FarA, ScbR, and ArpA) gave a 102-bp band. The sequence of this band had a high similarity to the expected region of a receptor gene. By genomic Southern hybridization with the 102-bp insert as a probe, a 687-bp intact receptor gene (sngR) was obtained from S. natalensis. To clarify the in vivo function of sngR, a sngR-disrupted strain was constructed, and the phenotypes were compared with those of the wild-type strain. The sngR-disruptants started natamycin production 6 h earlier and showed a 4.6-fold higher production of natamycin than the wild-type strain. In addition, the sporulation began earlier and the number of spores was tenfold more abundant than that of the wild-type strain. All the phenotypes were restored back to the original phenotypes of the wild-type strain by complementation with the intact sngR, indicating that the autoregulator receptor protein of S. natalensis acts as a primary negative regulator both on the biosynthesis of natamycin and sporulation.







Similar content being viewed by others
References
Aparicio JF, Colina AJ, Ceballos E, Martin JF (1999) The biosynthetic gene cluster for the 26-membered ring polyene macrolide pimaricin: a new polyketide synthase organization encoded by two subclusters separated by functionalization genes. J Biol Chem 274:10133–10139
Bibb MJ, Findlay PR, Johnson MW (1984) The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene 30:157–166
Bierman M, Logan R, O’Brien K, Seno ET, Rao RN, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Choi SU, Lee CK, Hwang YI, Kinoshita H, Nihira T (2004) Cloning and functional analysis by gene disruption of a gene encoding a γ-butyrolactone autoregulator receptor from Kitasatospora setae. J Bacteriol 186:3423–3430
Currie DC, Lueck C, Milburn HJ, Harvey C, Longbottom JL, Darbyshire JH, Nunn AN, Cole PJ (1990) Controlled trial of natamycin in the treatment of allergic bronchopulmonary aspergillosis. Thorax 45:447–450
Engel P, Scharfenstein LL, Dyer JM, Cary JW (2001) Disruption of a gene encoding a putative γ-butyrolactone-binding protein in Streptomyces tendae affects nikkomycin production. Appl Microbiol Biotechnol 56:414–419
Folcher M, Gaillard H, Nguyen LT, Nguyen KT, Lacroix P, Bamas-Jacques N, Rinkel M, Thompson CJ (2001) Pleiotropic functions of a Streptomyces pristinaespiralis autoregulator receptor in development, antibiotic biosynthesis, and expression of a superoxide dismutase. J Biol Chem 276:44297–44306
Grant SGN, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci USA 87:4645–4649
Hashimoto K, Nihira T, Yamada Y (1992) Distribution of virginiae butanolides and IM-2 in the genus Streptomyces. J Ferment Bioeng 73:61–65
Hobbs G, Frazer CM, Gardner DCJ, Cullum JA, Oliver SG (1989) Dispersed growth of Streptomyces in liquid culture. Appl Microbiol Biotechnol 31:272–277
Kiermeier F (1973) Zum einsatz von pimaricin zur verhinderung der schimmelpilz entwicklung auf lebensmitteln. Z Lebensm Unters Forsch 151:179–186
Kieser T, Bibb MJ, Buttner MJ, Chater KF Hopwood DA (2000) Practical Streptomyces genetics. The John Innes Foundation, Norwich
Kim HS, Nihira T Tada, Yanagimoto M, Yamada Y (1989) Identification of binding protein of virginiae butanolide C, an autoregulator in virginiamycin production, from Streptomyces virginiae. J Antibiot 42:796–778
Kim HS, Tada H, Nihira T, Yamada Y (1990) Purification and characterization of virginiae butanolide C-binding protein, a possible pleiotropic signal-transducer in Streptomyces virginiae. J Antibiot 43:692–706
Kinoshita H, Ipposhi H, Okamoto S, Nakano H, Nihira T, Yamada Y (1997) Butyrolactone autoregulator receptor protein (BarA) as a transcriptional regulator in Streptomyces virginiae. J Bacteriol 179:6986–6993
Kinoshita H, Tsuji T, Ipposhi H, Nihira T, Yamada Y (1999) Characterization of binding sequences for butyrolactone autoregulator receptors in streptomycetes. J Bacteriol 181:5075–5080
Kitani S, Kinoshita H, Nihira T, Yamada Y (1999) In vitro analysis of the butyrolactone autoregulator receptor protein (FarA) of Streptomyces lavendulae FRI-5 reveals that FarA acts as a DNA-binding transcriptional regulator that controls its own synthesis. J Bacteriol 181:5081–5084
Kitani S, Mervyn B, Nihira T, Yamada Y (2000) Conjugal transfer of plasmid DNA from Escherichia coli to Streptomyces lavendulae FRI-5. J Microbiol Biotechnol 10:535–538
Kitani S, Yamada Y, Nihira T (2001) Gene replacement analysis of the butyrolactone autoregulator receptor receptor (FarA) reveals that FarA acts as an novel regulator in secondary metabolism of Streptomyces lavendulae FRI-5. J Bacteriol 183:4357–4363
Kondo K, Higuchi Y, Sakuda S, Nihira T, Yamada Y (1989) New virginiae butanolide from Streptomyces virginiae. J Antibiot 42:1873–1876
Levinskas GJ, Ribelin WE, Shaffer CB (1966) Acute and chronic toxicity of pimaricin. Toxicol Appl Pharmacol 8:97–109
Mori K (1983) Revision of the absolute configuration of A-factor. Tetrahedron 39:3107–3109
Nakano H, Takehara E, Nihira T, Yamada Y (1998) Gene replacement analysis of the Streptomyces virginiae barA gene encoding the butyrolactone autoregulator receptor reveals that BarA acts as a repressor in virginiamycin biosynthesis. J Bacteriol 180:3317–3322
Ohashi H, Zheng YH, Nihira T, Yamada Y (1989) Distribution of virginiae butanolides in antibiotic-producing Actinomycetes, and identification of the inducing factor from Streptomyces antibioticus as virginiae butanolides A. J Antibiot 42:1191–1195
Ohnishi Y, Kameyama S, Onaka H, Horinouchi S (1999) The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: identification of a target gene of the A-factor receptor. Mol Microbiol 34:102–111
Okamoto S, Nakamura K, Nihira T, Yamada Y (1995) Virginiae butanolide binding protein from Streptomyces virginiae. J Biol Chem 270:12319–12326
Onaka H, Ando N, Nihira T, Yamada Y, Beppu T, Horinouchi S (1995) Cloning and characterization of the A-factor receptor gene from Streptomyces griseus. J Bacteriol 177:6083–6092
Onaka H, Sugiyama M, Horinouchi S (1997) A mutation at proline-115 in the A-factor receptor protein of Streptomyces griseus abolishes DNA-binding ability but not ligand-binding ability. J Bacteriol 179:2748–2752
Paranthaman S, Dharmalingam K (2003) Intergeneric conjugation in Streptomyces peucetius and Streptomyces sp. Strain C5: Chromosomal integration and expression of recombinant plasmids carrying the chiC gene. Appl Environ Microbiol 69:84–91
Pedersen JC (1992) Natamycin as a fungicide in agar media. Appl Environ Microbiol 58:1064–1066
Rao RN, Richardson MA, Kuhstoss S (1987) Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol 153:166–198
Ruengjitchatchawalya M, Nihira T, Yamada Y (1995) Purification and characterization of the IM-2-binding protein from Streptomyces sp. Strain FRI-5. J Bacteriol 177:551–557
Rusul G, Marth EH (1988) Growth and aflatoxin production by Aspergillus parasiticus in a medium at different pH values and with or without pimaricin. Z Lebensm Unters Forsch 187:436–439
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sato K, NihiraY, Sakuda S, Yanagimoto M, Yamada Y (1989) Isolation and structure of a new butyrolactone autoregulator from Streptomyces sp. FRI-5. J Ferment Bioeng 68:170–173
Stegmann E, Pelzer S, Wilken K, Wohlleben W (2001) Development of three different gene cloning systems for genetic investigation of the new species Amycolatopsis japonicum MG417-CF17, the ethylenediaminedisuccinic acid producer. J Biotechnol 92:195–204
Takano E, Nihira T, Hara Y, Jones JJ, Gershater CJL, Yamada Y, Bibb M (2000) Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2). J Biol Chem 275:11010–11016
Takano E, Chakraburtty R, Nihira T, Yamada Y, Bibb M (2001) A complex role for the γ-butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3(2). Mol Microbiol 41:1015–1028
Waki M, Nihira T, Yamada Y (1997) Cloning and Characterization of the gene (farA) encoding the receptor for an extracellular regulatory factor (IM-2) from Streptomyces sp. Strain FRI-5. J Bacteriol 179:5131–5137
Yamada Y, Sugamura K, Kondo K, Yanagimoto M, Okada H (1987) The structure of inducing factors for virginiamycin production in Streptomyces virginiae. J Antibiot 40:496–504
Acknowledgements
This work was supported by grant No. RTI04-03-07 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kang-Mu Lee and Chang-Kwon Lee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, KM., Lee, CK., Choi, SU. et al. Cloning and in vivo functional analysis by disruption of a gene encoding the γ-butyrolactone autoregulator receptor from Streptomyces natalensis . Arch Microbiol 184, 249–257 (2005). https://doi.org/10.1007/s00203-005-0047-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-005-0047-7