Abstract
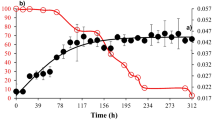
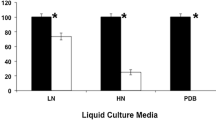
The efficiency of two lypolytic enzymes (fungal cutinase, yeast esterase) in the degradation of dipropyl phthalate (DPrP) was investigated. The DPrP-degradation rate of fungal cutinase was surprisingly high, i.e., almost 70% of the initial DPrP (500 mg/l) was decomposed within 2.5 h and nearly 50% of the degraded DPrP disappeared within the initial 15 min. With the yeast esterase, despite the same concentration, more than 90% of the DPrP remained even after 3 days of treatment. During the enzymatic degradation of DPrP, several DPrP-derived compounds were detected and time-course changes in composition were also monitored. The final chemical composition after 3 days was significantly dependent on the enzyme used. During degradation with fungal cutinase, most DPrP was converted into 1,3-isobenzofurandione (IBF) by diester hydrolysis. However, in the degradation by yeast esterase, propyl methyl phthalate (PrMP) was produced in abundance in addition to IBF. The toxic effects of the final degradation products were investigated using various recombinant bioluminescent bacteria. As a result, the degradation products (including PrMP) from yeast esterase severely caused oxidative stress and damage to protein synthesis in bacterial cells, while in the fungal cutinase processes, DPrP was significantly degraded to non-toxic IBF after the extended period (3 days).




Similar content being viewed by others
References
Belkin S, Smulski DR, Vollmer AC, Van Dyk TK, LaRossa RA (1996) Oxidative stress detection with Escherichia coli harboring a katG’::lux fusion. Appl Environ Microbiol 62:2252–2256
Cartwright CD, Owen SA, Thompson IP, Burns RG (2000) Biodegradation of diethyl phthalate in soil by a novel pathway. FEMS Microbiol Lett 18:27–34
Carvalho CM, Aires-Barros MR, Cabral JM (1999) Cutinase from molecular level to bioprocess development. Biotechnol Bioeng 66:17–34
Chang BV, Yang CM, Cheng CH, Yuan SY (2004) Biodegradation of phthalate esters by two bacteria strains. Chemosphere 55:533–538
Chatterjee S, Dutta TK (2003) Metabolism of butyl benzyl phthalate by Gordonia sp. strain MTCC 4818. Biochem Biophys Res Commun 309:36–43
Choi SH, Gu MB (2001) Phenolic toxicity-detection and classification through the use of a recombinant bioluminescent Escherichia coli. Environ Toxicol Chem 20:248–255
Dantzig AH, Zuckerman SH, Andonov-Roland MM (1986) Isolation of a Fusarium solani mutant reduced in cutinase activity and virulence. J Bacteriol 168:911–916
Davidov Y, Rozen R, Smulski DR, Van Dyk TK, Vollmer AC, Elsemore DA, LaRossa RA, Belkin S (2000) Improved bacterial SOS promoter:lux fusions for genotoxicity detection. Mutat Res 466:97–107
Flipsen J, Appel A, Van der Hijden H, Verrips C (1998) Mechanism of removal of immobilized triacylglycerol by lipolytic enzymes in a sequential laundry wash process. Enzyme Microb Technol 23:274–280
Gascon J, Qubina A, Barcelo D (1997) Detection of endocrinedisrupting pesticides by enzyme-linked immunosorbent assay (ELISA): application to atrazine. Trends Anal Chem 16:554–562
Gerard HC, Fett WF, Osman SF, Moreau RA (1993) Evaluation of cutinase activity of various industrial lipases. Biotechnol Appl Biochem 17:181–189
Gu MB, Min J, Kim EJ (2002) Toxicity monitoring and classification of endocrine-disrupting chemicals (EDCs) using recombinant bioluminescent bacteria. Chemosphere 46:289–294
Harris C, Henttu P, Parker M, Sumpter J (1997) The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105:802–811
Heindel JJ, Powell CJ (1992) Phthalate ester effects on rat Sertoli cell function in vitro. Toxicol Appl Pharmacol 115:116–123
Heindel JJ, Gulati DK, Mounce RC, Russell SR, Lamb JC (1989) Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundam Appl Toxicol 12:508–518
Kim YH, Lee J, Moon SH (2003) Uniqueness of microbial cutinase in hydrolysis of p-nitrophenyl esters. J Microbiol Biotechnol 13:57–63
Kim YH, Ahn JY, Moon SH, Lee J (2005) Biodegradation and detoxification of organophosphate insectcide, malathion by Fusarium oxysporum f. sp. pisi cutinase. Chemosphere (in press)
Kolattukudy PE (1984) Cutinase from fungi and pollen. In: Borgstrom B, Brockman T (eds) Lipases. Elsevier, Amsterdam, pp 471–504
Kolattukudy PE, Purdy RE, Maiti IB (1981) Cutinase from fungi and pollen. In: Lowenstein JM (eds) Methods in enzymology, vol 71. Academic, New York, pp 652–664
Lovekamp-Swan T, Davis BJ (2003) Mechanisms of Phthalate Ester Toxicity in the Female Reproductive System. Environ Health Perspect 111:139–145
Min J, Kim EJ, LaRossa RA, Gu MB (1999) Distinct responses of a recA::luxCDABE Escherichia coli strain to direct and indirect DNA damaging agents. Mutat Res 442:61–68
Murphy CA, Cameron JA, Huang SJ, Vinopal RT (1996) Fusarium polycaprolactone depolymerase is cutinase. Appl Environ Microbiol 62:456–460
Murphy CA, Cameron JA, Huang SJ, Vinopal RT (1998) A second polycaprolactone depolymerase from Fusarium, a lipase distant from cutinase. Appl Microbiol Biotechnol 50:692–696
Niazi JH, Prasad DT, Karegoudar TB (2001) Initial degradation of dimethylphthalate by esterases from Bacillusspecies. FEMS Microbiol Lett 96:201–205
Nunoshiba T, Nishioka H (1991) Rec-lac test for detecting SOS inducing activity of environmental genotoxic substances. Mutat Res 254:71–77
Okkels JS, Svendsen A, Borch K , Thellersen M, Patkar SA, Petersen DA, Royer JC, Kretzschmar T (1997) New lypolytic enzymes with high capacity to remove lard in one wash cycle. US patent 97–05735
Page BD, Lacroix GM (1992) Studies into the transfer and migration of phthalate esters from aluminium foil-paper laminates to butter and margarine. Food Addit Contam 9:197–212
Ptitsyn LR, Horneck G, Komova O, Kozubek S, Krasavin EA, Bonev M, Rettberg P (1997) A biosensor for environmental genotoxin screening based on an SOS lux assay in recombinant Escherichia coli cells. Appl Environ Microbiol 63:4377–4384
Purdy RE, Kolattukudy PE (1975) Hydrolysis of plant cuticle by plant pathogens. Purification, amino acid composition, and molecular weight of two isozymes of cutinase and a nonspecific esterase from Fusarium solanif. pisi. Biochemistry 14:2824–2831
Sagt CMJ, Muller WH, Boonstra J, Verkleij AJ, Verrips CT (1998) Impaired secretion of a hydrophobic cutinase by Saccharomyces cerevisiae correlates with an incerased association with immunoglobulin heavy-chain binding protein (BiP). Appl Environ Microbiol 64:316–324
Sebastian J, Chandra AK, Kolattukudy PE (1987) Discovery of a cutinase-producing Pseudomonas sp. cohabiting with an apparently nitrogen-fixing Corynebacterium sp. in phyllosphere. J Bacteriol 169:131–136
Shimao M (2001) Biodegradation of plastics. Curr Opin Biotechnol 12:242–247
Sung HH, Kao WY, Su YJ (2003) Effects and toxicity of phthalate esters to hemocytes of giant freshwater prawn, Macrobrachium rosenbergii. Aquat Toxicol 64:25–37
Suzuki T, Yaguchi K, Suzuki S, Suga T (2001) Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination. Environ Sci Technol 18:3757–3763
Unilever (1994) Enzyme-containing surfactant compositions. US patent 94–04771
Van Dyk TK, Majarian WR, Konstantinov KB, Young RM, Dhurjati PS, LaRossa RA (1994) Rapid and sensitive pollutant detection by induction of heat shock gene-bioluminescence gene fusions. Appl Environ Microbiol 60:1414–1420
Van Dyk TK, Smulski DR, Reed TR, Belkin S, Vollmer AC, LaRossa RA (1995) Responses to toxicants of an Escherichia coli strain carrying a uspA’::lux genetic fusion and an E. coli strain carrying a grpE’::lux fusion are similar. Appl Environ Microbiol 61:4124–4127
Vollmer AC, Belkin S, Smulski DR, Van Dyk TK, LaRossa RA (1997) Detection of DNA damage by use of Escherichia coli Carrying recA::lux, uvrA::lux, or alkA::lux reporter plasmids. Appl Environ Microbiol 63:2566–2571
Yuan SY, Liao CS, Liu C, Chang BV (2002) The occurrence and microbial degradation of phthalate esters of aquatic environmental in Taiwan. Chemosphere 49:1295–1299
Acknowledgements
We deeply thank Seung-Hyeon Moon at Gwangju Institute of Science and Technology for the GC/MS analysis used in this study. We are also grateful to Prof. C.M.J. Sagt in Utrecht University (Utrecht, Netherlands) for kindly providing the purified cutinase from F. oxysporum f. sp. pisi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YH., Min, J., Bae, KD. et al. Biodegradation of dipropyl phthalate and toxicity of its degradation products: a comparison of Fusarium oxysporum f. sp. pisi cutinase and Candida cylindracea esterase. Arch Microbiol 184, 25–31 (2005). https://doi.org/10.1007/s00203-005-0026-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-005-0026-z