Abstract
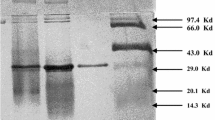
The thermoalkaliphilic anaerobic bacterium Anaerobranca gottschalkii produces an extracellular CGTase when grown on starch at 55°C and pH 9.0. The gene encoding this CGTase was cloned and successfully expressed in Escherichia coli. It encodes a protein consisting of 721 amino acids with a signal sequence of 34 amino acids. On SDS–polyacrylamide gels, the purified CGTase from A. gottschalkii displayed the expected molecular mass of 78 kDa. The recombinant enzyme was purified with a yield of 13.5% and displayed a specific activity of 210 units/mg. This CGTase, which represents the first report of a CGTase from an anaerobic thermoalkaliphile, was active at a broad range of temperature and pH, namely 55–70°C and pH 5–10. It completely converted amylose, amylopectin and native starch to cyclodextrins, preferentially α-cyclodextrin. With a longer incubation period, the α-cyclodextrin to β-cyclodextrin ratio declined. Variations in substrate type and concentration influenced the product pattern. Increasing the substrate concentration (0.5–20.0%) and glucans containing branching points (α-1,6 glycosidic linkages) shifted the product pattern to: β-cyclodextin > α-cyclodextrin > γ-cyclodextrin. In addition to these cyclodextrins, larger cyclodextrins (>8 glucose units) were formed in the initial reaction period. The CGTase was stabilised against thermal inactivation by calcium ions and high substrate concentrations; and 5 mM of CaCl2 shifted the apparent melting point of the enzyme from 60°C to 69°C.







Similar content being viewed by others
References
Biwer A, Antranikian G, Heinzle E (2002) Enzymatic production of cyclodextrins. Appl Microbiol Biotechnol 59:609–617
Bovetto LJ, Backer DP, Villette JR, Sicard PJ, Bouquelet SJ (1992) Cyclomaltodextrin glucanotransferase from Bacillus circulans E 192. I. Purification and characterization of the enzyme. Biotechnol Appl Biochem 15:48–58
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Britton HTS, Robinson RE (1931) Universal buffer solutions and the dissociation constants of veronal. J Chem Soc 458:1456–1473
Chung HJ, Yoon SH, Lee MJ, Kim MJ, Kweon KS, Lee IW, Kim JW, Oh BH, Lee HS, Spiridonova VA, Park KW (1998) Characterization of a thermostable cyclodextrin glucanotransferase isolated from Bacillus stearothermophilus ET1. J Agric Food Chem 46:952–959
Doukyu N, Kuwahara H, Aono R (2003) Isolation of Paenibacillus illinoisensis that produces cyclodextrin glucanotransferase resistant to organic solvents. Biosci Biotechnol Biochem 67:334–340
Endo T, Zheng M, Zimmermann W (2002) Enzymatic synthesis and analysis of large-ring cyclodextrins. Aust J Chem 55:39–48
Fiedler G, Pajatsch M, Bock A (1996) Genetics of a novel starch utilisation pathway present in Klebsiella oxytoca. J Mol Biol 256:279–291
Fujii K, Takata H, Yanase M, Terada Y, Ohdan K, Takaha T, Okada S, Kuriki T (2003) Bioengineering and application of novel glucose polymers. Biocatal Biotransform 21:167–172
Gawande BN, Patkar AY (1999) Application of factorial designs for optimization of cyclodextrin glycosyltransferase production from Klebsiella pneumoniae AS-22. Biotechnol Bioeng 64:168–173
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Hashimoto Y, Yamamoto T, Fujiwara S, Takagi M, Imanaka T (2001) Extracellular synthesis, specific recognition, and intracellular degradation of cyclomaltodextrins by the hyperthermophilic archaeon Thermococcus sp. strain B1001. J Bacteriol 183:5050–5057
Hungate RE (1969) A roll-tube method for the cultivation of strict anaerobes. Methods Microbiol 3B:117–132
Klein C, Schulz GE (1991) Structure of cyclodextrin glycosyltransferase refined at 2.0 Å resolution. J Mol Biol 217:737–750
Klenk HP, Ruepp A, Stark M, Zibat A, Becker I (2002) Screening for enzyme encoding genes in genomes of extremophilic prokaryotes. In: International congress on biocatalysis. Biocatalysis, Hamburg, p. 23
Koizumi K, Sanbe H, Kubota Y, Terada Y, Takaha T (1999) Isolation and characterization of cyclic alpha-(1→4)-glucans having degrees of polymerization 9–31 and their quantitative analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. J Chromatogr A 852:407–416
Krisman CR (1962) A method for the colorimetric estimation of glycogen with iodine. Anal Biochem 4:17–23
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larsen KL (2002) Large cyclodextrins. J Inclusion Phenom 43:1–13
Machida S, Ogawa S, Xiaohua S, Takaha T, Fujii K, Hayashi K (2000) Cycloamylose as an efficient artificial chaperone for protein refolding. FEBS Lett 486:131–135
Macy JM, Snellen JE, Hungate RE (1972) Use of syringe methods for anaerobiosis. Am J Clin Nutr 25:1318–1323
Martins RF, Hatti-Kaul R (2002) A new cyclodextrin glycosyltransferase from an alkaliphilic Bacillus agaradhaerens isolate: purification and characterisation. Enzyme Microb Technol 30:116–124
Martins RF, Hatti-Kaul R (2003) Bacillus agaradhaerens LS-3C cyclodextrin glycosyltransferase: activity and stability features. Enzyme Microb Technol 33:819–827
Matioli G, Zanin GM, De Moraes FF (2001) Characterization of cyclodextrin glycosyltransferase from Bacillus firmus strain no. 37. Appl Biochem Biotechnol 91–93:643–654
Matioli G, Zanin GM, De Moraes FF (2002) Influence of substrate and product concentrations on the production of cyclodextrins by CGTase of Bacillus firmus, strain no. 37. Appl Biochem Biotechnol 98–100:947–961
Nielsen JE, Borchert TV (2000) Protein engineering of bacterial α-amylases. Biochim Biophys Acta 1543:253–274
Nielsen H, Engelbrecht J, Brunak S, Heijne G von (1997) A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int J Neural Syst 8:581–599
Norman BE, Jørgensen ST (1992) Thermoanaerobacter sp. CGTase its properties and application. Denpun Kagaku 39:101–108
Pongsawasdi P, Yagisawa M (1988) Purification and some properties of cyclomaltodextrin glucanotransferases from Bacillus circulans. Agric Biol Chem 52:1099–1103
Prowe SG (1996) Characterization of extracellular amylolytic enzymes and sodium coupled energy transduction of a newly isolated thermoalkaliphilic bacterium Thermoalcalibacter bogoriae. Phd thesis, Technical University of Hamburg–Harburg, Hamburg
Prowe SG, Antranikian G (2001) Anaerobranca gottschalkii sp. nov., a novel thermoalkaliphilic bacterium that grows anaerobically at high pH and temperature. Int J Syst Evol Microbiol 51:457–465
Prowe SG, Vossenberg JL van de, Driessen AJ, Antranikian G, Konings WN (1996) Sodium-coupled energy transduction in the newly isolated thermoalkaliphilic strain LBS3. J Bacteriol 178:4099–4104
Rashid N, Cornista J, Ezaki S, Fukui T, Atomi H, Imanaka T (2002) Characterization of an archaeal cyclodextrin glucanotransferase with a novel C-terminal domain. J Bacteriol 184:777–784
Slominska L, Sobkowiak B (1997) Studies on cyclodextrin synthesis by novel cyclodextrin glycosyl transferase. Starch 7/8:301–305
Stoll VS, Blanchard JS (1990) Buffers: principles and practice. Methods Enzymol 182:24–38
Tachibana Y, Kuramura A, Shirasaka N, Suzuki Y, Yamamoto T, Fujiwara S, Takagi M, Imanaka T (1999) Purification and characterization of an extremely thermostable cyclomaltodextrin glucanotransferase from a newly isolated hyperthermophilic archaeon, a Thermococcus sp. Appl Environ Microbiol 65:1991–1997
Takada M, Nakagawa Y, Yamamoto M (2003) Biochemical and genetic analyses of a novel gamma-cyclodextrin glucanotransferase from an alkalophilic Bacillus clarkii 7364. J Biochem (Tokyo) 133:317–324
Terada Y, Yanase M, Takata H, Takaha T, Okada S (1997) Cyclodextrins are not the major cyclic alpha-1,4-glucans produced by the initial action of cyclodextrin glucanotransferase on amylose. J Biol Chem 272:15729–15733
Terada Y, Sanbe H, Takaha T, Kitahata S, Koizumi K, Okada S (2001) Comparative study of the cyclization reactions of three bacterial cyclomaltodextrin glucanotransferases. Appl Environ Microbiol 67:1453–1460
Tomita K, Kaneda M, Kawamura K, Nakanishi K (1993) Purification and properties of a cyclodextrin glucanotransferase from Bacillus autolyticus 11149 and selective formation of β-cyclodextrin. J Ferment Bioeng 75:89–92
Tonkova A (1998) Bacterial cyclodextrin glucanotransferase. Enzyme Microb Technol 22:678–686
Veen BA van der, Alebeek GJ van, Uitdehaag JC, Dijkstra BW, Dijkhuizen L (2000) The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem 267:658–665
Vikmon M (1982) Rapid and simple spectrophotometric method for determination of microamounts of cyclodextrins. In: Szejtli JD (ed) First international symposium on cyclodextrins. Reidel, Dordrecht, pp 69–74
Wind RD, Liebl W, Buitelaar RM, Penninga D, Spreinat A, Dijkhuizen L, Bahl H (1995) Cyclodextrin formation by the thermostable alpha-amylase of Thermoanaerobacterium thermosulfurigenes EM1 and reclassification of the enzyme as a cyclodextrin glycosyltransferase. Appl Environ Microbiol 61:1257–1265
Acknowledgement
We thank the Deutsche Bundesstiftung Umwelt (German Federal Environmental Foundation) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Hans G. Schlegel on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Thiemann, V., Dönges, C., Prowe, S.G. et al. Characterisation of a thermoalkali-stable cyclodextrin glycosyltransferase from the anaerobic thermoalkaliphilic bacterium Anaerobranca gottschalkii. Arch Microbiol 182, 226–235 (2004). https://doi.org/10.1007/s00203-004-0717-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-004-0717-x