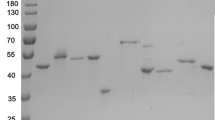
Abstract
Methionine aminopeptidase, known to be encoded by single genes in prokaryotes, is a cobalt-dependent enzyme that catalyzes the removal of N-terminal methionine residues from nascent polypeptides. Three ORFs encoding putative methionine aminopeptidases from the genome of cyanobacterium Synechocystis sp. strain PCC6803, designated as slr0786 (map-1), slr0918 (map-2) and sll0555 (map-3) were cloned and expressed in Escherichia coli. The purified recombinant proteins encoded by map-1 and map-3 had much higher methionine aminopeptidase activity than the recombinant protein encoded by map-2. Comparative analysis revealed that the three recombinant enzymes differed in their substrate specificity, divalent ion requirement, pH, and temperature optima. The broad activities of the iso-enzymes are discussed in light of the structural similarities with other peptidase families and their levels of specificity in the cell. Potential application of cyanobacterial MetAPs in the production of recombinant proteins used in medicine is proposed. This is the first report of a prokaryote harboring multiple methionine aminopeptidases.




Similar content being viewed by others
Abbreviations
- map :
-
Gene encoding methionine aminopeptidase
- MetAP :
-
Methionine aminopeptidase
- eMetAP-Ia :
-
Escherichia coli methionine aminopeptidase type Ia
- yMetAP-Ib :
-
Yeast methionine aminopeptidase type Ib
- yMetAP-IIa :
-
Yeast methionine aminopeptidase type IIa
- hMetAP-IIb :
-
Human methionine aminopeptidase type IIb
- pfMetAP−IIa :
-
Pyrococcus furiosis methionine aminopeptidase type Ia
- bst MetAP-Ia :
-
Bacillus stearothermophilus methionine aminopeptidase type Ia
- c1MetAP-Ia :
-
Cyanobacterial methionine aminopeptidase type Ia encoded by map-1
- c2MetAP-Ia :
-
Cyanobacterial methionine aminopeptidase type Ia encoded by map-2
- c3MetAP-Ib :
-
Cyanobacterial methionine aminopeptidase type Ib, ncoded by map-3
References
Allen G (1981) Determination of peptide sequences. In: Work TS, Burdon RH (eds) Sequencing of proteins and peptides. Elsevier, Amsterdam, pp. 13–136,174–188
Arfin SM, Kendall RL, Hall L, Weaver LH, Stewart AE, Matthews BW, Bradshaw RA (1995) Eukaryotic methionyl aminopeptidases: two classes of cobalt-dependent enzymes. Proc Natl Acad Sci USA 9:7714–7718
Bazan JF, Weaver LH, Roderick SL, Huber R, Matthews BW. (1994) Sequence and structure comparison suggest that methionine aminopeptidase, prolidase, aminopeptidase P, and creatinase share a common fold. Proc Natl Acad Sci USA 91:2473–2477
Ben-Bassat A, Bauer K, Chang SY, Myambo K, Boosman A, Chang S (1987) Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol 169:751–577
Brahamsha B, Haselkorn R (1992) Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: cloning, expression, and inactivation of the sigB and sigC genes. J Bacteriol 174:7273–7282
Chang Y-H, Teichert U, Smith JA (1992) Molecular cloning, sequencing, deletion, and overexpression of a methionine aminopeptidase gene from Saccharomyces cerevisiae. J Biol Chem 267:8007–8011
Chen S, Vetro JA, Chang YH (2002) The specificity in vivo of two distinct methionine aminopeptidases in Saccharomyces cerevisiae. Arch Biochem Biophys 398:87–93
Chung JM, Chung IY, Lee YS. (2002) The purification and characterization of a Bacillus stearothermophilus methionine aminopeptidase (MetAP). J Biochem Mol Biol 35:228–235
Damerval T, Castets AM, Guglielmi G, Houmard J, Tandeau de Marsac N (1989) Occurrence and distribution of gas vesicle genes among cyanobacteria. J Bacteriol 171:1445–1452
D'souza VM, Holz RC (1999) The methionyl aminopeptidase from Escherichia coli can function as an iron(II) enzyme. Biochemistry 38:11079–11085
D'souza VM, Swierczek SI, Cosper NJ, Meng L, Ruebush S, Copik AJ, Scott RA, Holz RC. (2002) Kinetic and structural characterization of manganese(II)-loaded methionyl aminopeptidases. Biochemistry 41:13096–13105
Giglione C, Serero A, Pierre M, Boisson B, Meinnel T (2000) Identification of eukaryotic peptide deformylases reveals universality of N-terminal protein processing mechanisms. EMBO J 19:5916–5929
Griffith EC, Su Z, Turk BE, Chen S, Chang YH, Wu Z, Biemann K, Liu JO (1997) Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol 4:461–471
Howell ML, Blumenthal KM (1991) Mutagenesis of Cerebratulus lacteus neurotoxin B-IV identifies NH2-terminal sequences important for biological activity. J Biol Chem 266:12884–12888
Jorgensen AT, Norrby PO, Liljefors T (2002) Investigation of the metal binding site in methionine aminopeptidase by density functional theory. J Comput Aided Mol Des 16:167–179
Joseph-Liauzun E, Leplatois P, Legoux R, Guerveno V, Marchese E, Ferrara P (1990) Human recombinant interleukin-1 beta isolated from Escherichia coli by simple osmotic shock. Gene 8:291–295
Kabashima T, Kitazono A, Kitano A, Ito K, Yoshimoto T (1997) Prolyl aminopeptidase from Serratia marcescens: cloning of the enzyme gene and crystallization of the expressed enzyme. J Biochem 122:601–605
Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions (supplement). DNA Research 3:185–209
Klein JR, Dick A, Schick J, Matern HT, Henrich B, Plapp R (1995) Molecular cloning and DNA sequence analysis of pepL, a leucyl aminopeptidase gene from Lactobacillus delbrueckii subsp. lactis DSM7290. Eur J Biochem 22:570–578
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 22:680–685
Li X, Chang Y-H (1995) Amino-terminal protein processing in Saccharomyces cerevisiae is an essential function that requires two distinct methionine aminopeptidases. Proc Natl Acad Sci USA 92:12357–12361
Li X, Chang Y-H (1996) Evidence that the human homologue of a rat initiation factor-2 associated protein (p67) is a methionine aminopeptidase. Biochem Biophys Res Comm 227:152–159
Liu S, Widom J, Kemp CW, Crews CM, Clardy J. (1998) Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science 282:1324–1327
Lowther WT, Matthews BW (2000) Structure and function of the methionine aminopeptidases. Biochim Biophys Acta 1477:157–167
Lowther WT, McMillen DA, Orville AM, Matthews BW (1998) The anti-angiogenic agent fumagillin covalently modifies a conserved active-site histidine in the Escherichia coli methionine aminopeptidase. Proc Natl Acad Sci USA 95:12153–12157
Lowther WT, Orville AM, Madden DT, Lim S, Rich DH, Matthews BW (1999a) Escherichia coli methionine aminopeptidase: implications of crystallographic analyses of the native, mutant, and inhibited enzymes for the mechanism of catalysis. Biochemistry 3:7678–7688
Lowther WT, Zhang Y, Sampson PB, Honek JF, Matthews BW (1999b) Insights into the mechanism of Escherichia coli methionine aminopeptidase from the structural analysis of reaction products and phosphorus-based transition-state analogues. Biochemistry 38:14810–14819
Moreira D, Philippe H (2001) Sure facts and open questions about the origin and evolution of photosynthetic plastids. Res Microbiol 152:771–780
Nakamura K, Tanaka T, Kuwahara A, Takeo K (1985) Microassay for proteins on nitrocellulose filter using protein dye-staining procedure. Anal Biochem 148:311–319
Oja VV, Savchenko G, Jakob B, Heber U (1999) pH and buffer capacities of apoplastic and cytoplasmic cell compartments in leaves. Planta 209:239–249
Patterson EK Gatmaitan JS, Hayman S (1973) Substrate specificity and pH dependence of dipeptidases purified from Escherichia coli B and from mouse ascites tumor cells. Biochemistry 12:3701–3709
Priestman DA, Butterworth J (1985) Prolinase and non-specific dipeptidase of human kidney. Biochem J 231:689–694
Proudfoot AE, Power CA, Hoogewerf AJ, Montjovent MO, Borlat F, Offord RE, Wells TN (1996) Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem 271:2599–2603
Roderick SL, Matthews BW (1993) Structure of the cobalt-dependent methionine aminopeptidase from Escherichia coli: a new type of proteolytic enzyme. Biochemistry 32:3907–3912
Rawlings ND, Barrett AJ (1995) Evolutionary families of metallopeptidases. Meth Enzymol 248:183–228
Sedo A, Malik R. (2001) Dipeptidyl peptidase IV-like molecules: homologous proteins or homologous activities? Biochim Biophys Acta 1550:107–116
Sin N, Meng L, Wang M, Wen J, Bornmann W, Crews C (1997) The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase MetAP-2. Proc Natl Acad Sci USA 94:6099–6103
Sugiura M, Hirose T, Sugita M (1998) Evolution and mechanism of translation in chloroplasts. Annu Rev Genet 32:437–459
Tahirov TH, Oki H, Tsukihara T, Ogasahara K, Yutani K, Ogata K, Izu Y, Tsunasawa S, Kato I (1998) Crystal structure of methionine aminopeptidase from hyperthermophile, Pyrococcus furiosus. J Mol Biol 284:101–124
Tanaka K, Masuda S, Takahashi H (1992) Multiple rpoD-related genes of cyanobacteria. Biosci Biotechnol Biochem 56:1113–1117
Taroncher-Oldenburg G, Anderson DM (2000) Identification and characterization of three differentially expressed genes, encoding S-adenosylhomocysteine hydrolase, methionine aminopeptidase, and a histone-like protein, in the toxic dinoflagellate Alexandrium fundyense. Appl Environ Microbiol 66:2105–2112
Tsunasawa S, Izu Y, Miyagi M, Kato I (1997) Methionine aminopeptidase from the hyperthermophilic Archaeon Pyrococcus furiosus: molecular cloning and overexpression in Escherichia coli of the gene, and characteristics of the enzyme. J Biochem 122:843–850
Turk B, Griffith E, Wolf S, Biemann K, Chang Y, Liu J (1999) Selective inhibition of amino-terminal methionine processing by TNP-470 and ovalicin in endothelial cells. Chem Biol 6:823–833
Varshavsky A (1997) The N-end rule pathway of protein degradation. Genes Cells 2:13–28
Walker KW, Bradshaw RA (1998) Yeast methionine aminopeptidase I can utilize either Zn2+ or Co2+ as a cofactor: a case of mistaken identity? Protein Sci 7:2684–2687
Walker KW, Bradshaw RA (1999) Yeast methionine aminopeptidase I. Alteration of substrate specificity by site-directed mutagenesis. J Biol Chem 274:13403–13409
Wingfield P, Graber P, Turcatti G, Movva NR, Pelletier M, Craig S, Rose K, Miller CG (1989) Purification and characterization of a methionine-specific aminopeptidase from Salmonella typhimurium. Eur J Biochem 180:23–32
Yang G, Kirkpatrick RB, Ho T, Zhang GF, Liang PH, Johanson KO, Casper DJ, Doyle ML, Marino JP Jr, Thompson SK, Chen W, Tew DG, Meek TD (2001) Steady-state kinetic characterization of substrates and metal-ion specificities of the full-length and N-terminally truncated recombinant human methionine aminopeptidases (type 2). Biochemistry 40:10645–10654
Zuo S, Guo Q, Ling C, Chang YH (1995) Evidence that two zinc fingers in the methionine aminopeptidase from Saccharomyces cerevisiae are important for normal growth. Mol Gen Genet 246:247–253
Acknowledgements
This work was supported by a fellowship from the Japanese Society for the Promotion of Science and partly by a grant from the Bulgarian National Research Fund (K-609/1997). We thank Professor Deborah Zamble, Alistair Dias, Daria Yu, Teodora Jivkova, Asuka Mase, Nickolay Tzvetkov, Svetoslav Dimov, Dr. Igor Splawski and Dr. Michinori Mutsuda for help and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atanassova, A., Sugita, M., Sugiura, M. et al. Molecular cloning, expression and characterization of three distinctive genes encoding methionine aminopeptidases in cyanobacterium Synechocystis sp. strain PCC6803. Arch Microbiol 180, 185–193 (2003). https://doi.org/10.1007/s00203-003-0576-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-003-0576-x