Abstract
Summary
This post hoc analysis and modeling study examined the mechanism of action of odanacatib using a statistical model to explain sCTx response in ODN-treated patients as a function of other bone-turnover biomarkers that, with other observed biomarker changes, showed that odanacatib persistently inhibited osteoclastic bone removal activity without preventing osteoclastogenesis.
Introduction
Odanacatib (ODN) is an oral selective cathepsin K (CatK) inhibitor, previously in development for osteoporosis treatment. A post hoc analysis examined ODN’s mechanism of action on bone-turnover biomarkers.
Methods
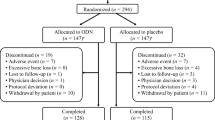
A subset of patients who completed 60 months’ treatment in the Long-Term Odanacatib Fracture Trial (LOFT; NCT00529373) (N = 112 [57 ODN, 55 placebo]) were evaluated. Serum (s) and urine (u) samples were assayed at baseline and months 6–60 for 10 known bone-remodeling biomarkers: sCTx, uαα- and uββCTx/Cr, uNTx/Cr, sNTx, uDPD/Cr, sICTP, sTRAP5b, sPINP, and sBSAP. Because the CrossLaps® CTx assay identifies the CTx peptide as well as larger molecular weight CTx-containing peptides, including ICTP, a best-fit model was developed to explain the transient sCTx reduction in ODN-treated patients.
Results
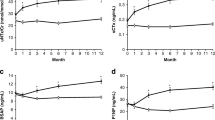
ODN persistently reduced the bone-resorption markers sNTx, uNTx/Cr, uαα- and uββCTx/Cr, and uDPD/Cr, and gradually increased the target-engagement marker sICTP and osteoclast number (sTRAP5b), versus placebo from baseline to month 60. sCTx was transiently reduced with ODN within 12 months, returning to baseline by month 48. Modeling suggested that sCTx changes in the ODN group were primarily due to increased accumulation of larger CTx species, including sICTP. The bone-formation markers sPINP and sBSAP showed partial reductions, versus placebo, in the first 6 months but approached baseline by months 48–60.
Conclusion
Observed changes in bone-turnover biomarkers support the persistent efficacy of ODN in direct inhibition of osteoclastic bone-resorption activity, without inhibition of osteoclastogenesis. Long-term evaluation also underscores the unique mechanism of ODN on osteoclastic collagen processing and subsequently osteoblastic bone formation.
Trial registration
NCT00529373.




Similar content being viewed by others
Data availability
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Code availability
Not applicable.
References
NIH Consensus Development Panel (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Bone HG, Dempster DW, Eisman JA et al (2015) Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the Long-Term Odanacatib Fracture Trial. Osteoporos Int 26:699–712
McClung MR, O’Donoghue ML, Papapoulos SE et al (2019) Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol 7:899–911
Kuo TR, Chen CH (2017) Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res 5:18
Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaisse JM (2003) The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res 18:859–867
Garnero P (2008) Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther 12:157–170
Yamauchi M, Sricholpech M (2012) Lysine post-translational modifications of collagen. Essays Biochem 52:113–133
Cloos PA, Fledelius C, Christgau S, Christiansen C, Engsig M, Delmas P, Body JJ, Garnero P (2003) Investigation of bone disease using isomerized and racemized fragments of type I collagen. Calcif Tissue Int 72:8–17
Fledelius C, Johnsen AH, Cloos PA, Bonde M, Qvist P (1997) Characterization of urinary degradation products derived from type I collagen. Identification of a beta-isomerized Asp-Gly sequence within the C-terminal telopeptide (alpha1) region. J Biol Chem 272:9755–9763
Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaisse JM (1998) The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273:32347–32352
Sassi ML, Eriksen H, Risteli L, Niemi S, Mansell J, Gowen M, Risteli J (2000) Immunochemical characterization of assay for carboxyterminal telopeptide of human type I collagen: loss of antigenicity by treatment with cathepsin K. Bone 26:367–373
Duong LT (2012) Therapeutic inhibition of cathepsin K - reducing bone resorption while maintaining bone formation. Bonekey Rep 1:67
Beardsworth LJ, Eyre DR, Dickson IR (1990) Changes with age in the urinary excretion of lysyl- and hydroxylysylpyridinoline, two new markers of bone collagen turnover. J Bone Miner Res 5:671–676
Colwell A, Eastell R (1996) The renal clearance of free and conjugated pyridinium cross-links of collagen. J Bone Miner Res 11:1976–1980
Crofton PM, Evans N, Taylor MR, Holland CV (2002) Serum CrossLaps: pediatric reference intervals from birth to 19 years of age. Clin Chem 48:671–673
Immunodiagnostic Systems (2017) Serum Crosslaps® (CTX-I) ELISA. http://www.idsplc.com/products/serum-crosslaps-ctx-i-elisa-2/. Accessed 16 May 2022
Leung P, Pickarski M, Zhuo Y, Masarachia PJ, Duong LT (2011) The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 49:623–635
Vonesh EF (2014) Chapter 5. Generalized linear and nonlinear mixed-effects models. In: Generalized linear and nonlinear models for correlated data: theory and applications using SAS. Cary, NC, SAS Institute, pp 205–332
Eastell R, Mallinak N, Weiss S, Ettinger M, Pettinger M, Cain D, Fressland K, Chesnut C 3rd (2000) Biological variability of serum and urinary N-telopeptides of type I collagen in postmenopausal women. J Bone Miner Res 15:594–598
Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD (1999) Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 14:1614–1621
Garnero P, Fledelius C, Gineyts E, Serre CM, Vignot E, Delmas PD (1997) Decreased beta-isomerization of the C-terminal telopeptide of type I collagen alpha 1 chain in Paget’s disease of bone. J Bone Miner Res 12:1407–1415
Zaitseva OV, Shandrenko SG, Veliky MM (2015) Biochemical markers of bone collagen type I metabolism. Ukr Biochem J 87:21–32
Recker R, Dempster D, Langdahl B et al (2020) Effects of odanacatib on bone structure and quality in postmenopausal women with osteoporosis: 5-year data from the phase 3 long-term odanacatib fracture trial (LOFT) and its extension. J Bone Miner Res 35:1289–1299
Eisman JA, Bone HG, Hosking DJ et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Langdahl B, Binkley N, Bone H et al (2012) Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res 27:2251–2258
Pennypacker BL, Gilberto D, Gatto NT, Samadfam R, Smith SY, Kimmel DB, Thi DL (2016) Odanacatib increases mineralized callus during fracture healing in a rabbit ulnar osteotomy model. J Orthop Res 34:72–80
Acknowledgements
Medical writing assistance, under the direction of the authors, was provided by Gráinne Faherty, MPharm, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The study sponsor was involved in the study design, collection, analysis and interpretation of data, and writing of the report. Although a clearance review of this manuscript was performed by the sponsor to protect intellectual property or competitive information and prevent inadvertent disclosure, all authors had access to the study data, contributed to the development of this manuscript, approved content, and agreed with the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable, post hoc analysis.
Consent to participate
Not applicable, post hoc analysis.
Consent for publication
Not applicable, data anonymized.
Conflict of interest
Le T. Duong, Seth Clark, Maureen Pickarski, Hilde Giezek, Dosinda Cohn, Rachid Massaad, and S. Aubrey Stoch are, or were at the time of study conduct, employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or hold stock options in the Company.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duong, L.T., Clark, S., Pickarski, M. et al. Effects of odanacatib on bone-turnover markers in osteoporotic postmenopausal women: a post hoc analysis of the LOFT study. Osteoporos Int 33, 2165–2175 (2022). https://doi.org/10.1007/s00198-022-06406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06406-x