Abstract
Summary
TRACP-5b can be used to monitor the response of treatments in osteoporosis. We investigated the effect of feeding on levels of TRACP-5b and how these markers perform in a clinical setting. After feeding, there was no effect on levels TRACP-5b. It has similar diagnostic accuracy to CTX and PINP.
Introduction
Bone turnover markers (BTMs) can be used to monitor response to osteoporosis treatment. However, some are affected by food intake and are not suitable to measure in a clinical setting. An assay is available which is capable of detecting the active isoform 5b of tartrate resistance acid phosphatase (TRACP-5b) and it may have minimal biological variation. Our aims were to investigate the effect of feeding on levels of TRACP-5b and compare this to CTX and PINP and then to compare the diagnostic accuracy of TRACP-5b to CTX and PINP in patients with osteoporosis given commonly used treatments.
Methods
Eighteen patients were recruited to investigate the effect of feeding on BTMs. Ninety-seven patients (74 females and 23 males) receiving 5 mg annual intra-venous zoledronate (mean age 70) and 97 patients receiving no treatment were recruited as group-matched controls. Sixteen patients receiving 60 mg subcutaneous denosumab every 6 months, (mean age 76) and 16 matched controls were recruited. Seventy-six patients were receiving oral bisphosphonates: 70 mg alendronate weekly, 35 mg risedronate and 150 mg monthly ibandronate (4%). Thirty of these patients had BMD measured at the total hip and lumbar spine. An estimate of compliance was not determined. Eighty patients receiving no treatment were recruited as group-matched controls. TRACP-5b (ELISA, Nittobo) and CTX and PINP were measured in serum in the non-fasting state between 0800 and 1700.
Results
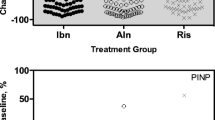
After feeding, there was no effect on levels TRACP-5b and significant reductions in CTX and PINP, 29% and 10%, respectively (p < 0.001). In the zoledronate and denosumab groups, there were no differences in the areas under the curves (AUCs) between TRACP-5b, PINP and CTX. In the oral bisphosphonates group, the AUCs between TRACP-5b and PINP and TRACP-5b and CTX were significantly different, p < 0.01 and p = 0.001, respectively. TRACP-5b was negatively correlated with BMD.
Conclusion
TRACP-5b is not affected by food intake, unlike CTX and PINP. All three BTMs correlate with change in BMD at the lumbar spine and total hip. TRACP-5b has similar diagnostic accuracy to CTX and PINP with commonly used treatments for osteoporosis with the exception of oral bisphosphonate therapy.


Similar content being viewed by others
References
Eastell R, Szulc P (2017) Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol 5(11):908–923
Eastell R et al (2018) DIAGNOSIS OF ENDOCRINE DISEASE: Bone turnover markers: are they clinically useful? Eur J Endocrinol 178(1):R19-r31
Vasikaran S et al (2011) International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med 49(8):1271–1274
Diez-Perez A et al (2017) International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int 28(3):767–774
Szulc P et al (2017) Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28(9):2541–2556
Redmond J et al (2016) Diurnal rhythms of bone turnover markers in three ethnic groups. J Clin Endocrinol Metab 101(8):3222–3230
Hannon RA et al (2004) Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone 34(1):187–194
Walsh JS, Henriksen DB (2010) Feeding and bone. Arch Biochem Biophys 503(1):11–19
Yaziji H et al (1995) Immunohistochemical detection of tartrate-resistant acid phosphatase in non-hematopoietic human tissues. Am J Clin Pathol 104(4):397–402
Halleen JM et al (2006) Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 52(9–10):499–509
Lam KW et al (1981) Comparison of the tartrate-resistant acid phosphatase in Gaucher’s disease and leukemic reticuloendotheliosis. Clin Biochem 14(4):177–181
Ohashi T et al (2007) Development of a novel fragments absorbed immunocapture enzyme assay system for tartrate-resistant acid phosphatase 5b. Clin Chim Acta 376(1–2):205–212
Eastell R et al (2011) Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res 26(3):530–537
Paggiosi MA et al (2014) Comparison of the effects of three oral bisphosphonate therapies on the peripheral skeleton in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 25(12):2729–2741
Clowes JA et al (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30(6):886–890
Ju HS et al (1997) Comparison of analytical performance and biological variability of three bone resorption assays. Clin Chem 43(9):1570–1576
Schlemmer A et al (1992) Marked diurnal variation in urinary excretion of pyridinium cross-links in premenopausal women. J Clin Endocrinol Metab 74(3):476–480
Yu EW et al (2016) Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab 101(2):714–722
Fuglsang-Nielsen R et al (2020) Consumption of nutrients and insulin resistance suppress markers of bone turnover in subjects with abdominal obesity. Bone 133:115230
Shapses SA, Sukumar D (2012) Bone metabolism in obesity and weight loss. Annu Rev Nutr 32:287–309
Kreitman A et al (2021) Reduced postprandial bone resorption and greater rise in GLP-1 in overweight and obese individuals after an α-glucosidase inhibitor: a double-blinded randomized crossover trial. Osteoporos Int 32(7):1379–1386
Morley J et al (2019) Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int
Fatoye F et al (2019) Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 9(4):e027049
Lorentzon M et al (2019) Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther 36(10):2811–2824
Naylor KE et al (2016) Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 27(1):21–31
Cramer JA et al (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18(8):1023–1031
Stepan JJ et al (1983) Relationship of plasma tartrate resistant acid phosphatase to the bone isoenzyme of serum alkaline phosphatase in hyperparathyroidism. Clin Chim Acta 133(2):189–200
Szulc P (2018) Bone turnover: biology and assessment tools. Best Pract Res Clin Endocrinol Metab 32(5):725–738
Szulc P et al (2018) Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Ann Biol Clin (Paris) 76(4):373–391
Christgau S (2000) Circadian variation in serum CrossLaps concentration is reduced in fasting individuals. Clin Chem 46(3):431
Gossiel F et al (2014) Establishing reference intervals for bone turnover markers in healthy postmenopausal women in a nonfasting state. Bonekey Rep 3:573
Glover SJ et al (2009) Establishing a reference interval for bone turnover markers in 637 healthy, young, premenopausal women from the United Kingdom, France, Belgium, and the United States. J Bone Miner Res 24(3):389–397
Nishizawa Y et al (2008) Reference intervals of serum tartrate-resistant acid phosphatase type 5b activity measured with a novel assay in Japanese subjects. J Bone Miner Metab 26(3):265–270
Nakamura Y et al (2017) Two-year clinical outcome of denosumab treatment alone and in combination with teriparatide in Japanese treatment-naive postmenopausal osteoporotic women. Bone Res 5:16055
Ivaska KK et al (2008) Serial assessment of serum bone metabolism markers identifies women with the highest rate of bone loss and osteoporosis risk. J Clin Endocrinol Metab 93(7):2622–2632
Nenonen A et al (2005) Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. J Bone Miner Res 20(10):1804–1812
Nakatoh S (2018) Effect of osteoporosis medication on changes in bone mineral density and bone turnover markers after 24-month administration of daily teriparatide: comparison among minodronate, raloxifene, and eldecalcitol. J Bone Miner Metab 36(2):221–228
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R Eastell receives consultancy funding from IDS, Sandoz, Nittobo, Samsung, Haoma Medica, CL Bio, Biocon, Amgen, Hindustan Unilever, Pharmacosmos, Takeda and Viking and grant funding from Nittobo, Roche, Pharmacosmos and Alexion. J Walsh has received speaker’s honoraria from Lilly and the donation of drug and placebo from Prostrakan. N Peel has received speaker’s honoraria and funding to attend educational events from Warner-Chilcott, Lilly, Amgen, GSK and Prostrakan and consultancy fees from Internis Pharma and Lilly. F Gossiel and A Ugar declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gossiel, F., Ugur, A., Peel, N.F.A. et al. The clinical utility of TRACP-5b to monitor anti-resorptive treatments of osteoporosis. Osteoporos Int 33, 1357–1363 (2022). https://doi.org/10.1007/s00198-022-06311-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06311-3