Abstract
Summary
To identify the critical genes and pathways that related to OP development in male AS patients, bioinformatic gene analysis and qRT-PCR validation were performed. SBNO2 and VPS13B were identified as the potential target for OP development, which may be valuable for the prevention of OP in male AS patients.
Introduction
Osteoporosis (OP) is common in men with ankylosing spondylitis (AS). The specific pathogenesis of OP in AS, however, is still unclear. The present study attempted to identify potential genes associated with the development of OP in males with AS.
Methods
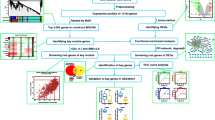
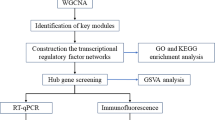
Gene expression profiles were downloaded from the GSE73754 and GSE35959 datasets from the Gene Expression Omnibus (GEO). Data from OsteoporosAtlas were downloaded as a supplement. Differentially expressed genes (DEGs) were determined with the limma package. The overlapping DEGs between male AS-related genes and OP-related genes were determined. The DEGs were validated by qRT-PCR in the blood samples of males with AS. Weighted gene co-expression network analysis (WGCNA) was utilized to establish a co-expression network to identify the hub genes.
Results
A total of 17 overlapping DEGs were identified; 6 genes in 17 overlapping DEGs were verified as the essential genes in the pathogenesis of OP in male AS by qRT-PCR analysis. After WGCNA, the modules of MEblue (> 0.6) and MEred (> 0.8) were screened out by the correlation analysis and were determined to function mainly in MAPK signaling pathway and osteoclast differentiation. Analysis of the two modules revealed VPS13B and SBNO2 as key genes due to the high degree of correlation. Both genes play an important role in bone metabolism regulation in male AS. Two hub genes MYD88 in MEblue and NCK1 in MEred with high degree of connectivity were selected.
Conclusions
Gender-specific SBNO2 and VPS13B may be key genes involved in OP in male AS.






Similar content being viewed by others
Abbreviations
- OP:
-
Osteoporosis
- AS:
-
Ankylosing spondylitis
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differential expression genes
- qRT-PCR:
-
Quantitative reverse transcriptase-polymerase chain reaction
- BMD:
-
Bone mineral density
- WGCNA:
-
Construction of weighted gene co-expression network analysis
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- FNCSGPs:
-
Femoral neck cross-sectional geometric parameters
- TAL1:
-
T cell acute lymphoblastic leukemia 1
- MITF:
-
Microphthalmia-associated transcription factor
- MAPK:
-
Mitogen-activated protein kinase
- BMP:
-
Bone morphogenetic protein
- DC-STAMP:
-
Dendritic cell-specific transmembrane protein
References
Klingberg E, Nurkkala M, Carlsten H, Forsblad-d’Elia H (2014) Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol 41:1349–1356
Bessant R, Keat A (2002) How should clinicians manage osteoporosis in ankylosing spondylitis? J Rheumatol 29:1511–1519
Magrey M, Khan MA (2010) Osteoporosis in ankylosing spondylitis. Curr Rheumatol Rep 12:332–336
Devogelaer JP, Maldague B, Malghem J, Nagant de Deuxchaisnes C (1992) Appendicular and vertebral bone mass in ankylosing spondylitis. A comparison of plain radiographs with single- and dual-photon absorptiometry and with quantitative computed tomography. Arthritis Rheum 35:1062–1067
Vasdev V, Bhakuni D, Garg MK, Narayanan K, Jain R, Chadha D (2011) Bone mineral density in young males with ankylosing spondylitis. Int J Rheum Dis 14:68–73
Karberg K, Zochling J, Sieper J, Felsenberg D, Braun J (2005) Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol 32:1290–1298
Lange U, Kluge A, Strunk J, Teichmann J, Bachmann G (2005) Ankylosing spondylitis and bone mineral density--what is the ideal tool for measurement? Rheumatol Int 26:115–120
Magrey MN, Lewis S, Asim Khan M (2016) Utility of DXA scanning and risk factors for osteoporosis in ankylosing spondylitis-a prospective study. Semin Arthritis Rheum 46:88–94
Hu LY, Lu T, Chen PM, Shen CC, Hung YM, Hsu CL (2019) Should clinicians pay more attention to the potential underdiagnosis of osteoporosis in patients with ankylosing spondylitis? A national population-based study in Taiwan. PLoS One 14:e0211835
Meirelles ES, Borelli A, Camargo OP (1999) Influence of disease activity and chronicity on ankylosing spondylitis bone mass loss. Clin Rheumatol 18:364–368
Franck H, Meurer T, Hofbauer LC (2004) Evaluation of bone mineral density, hormones, biochemical markers of bone metabolism, and osteoprotegerin serum levels in patients with ankylosing spondylitis. J Rheumatol 31:2236–2241
Wang X, Diao L, Sun D, Wang D, Zhu J, He Y, Liu Y, Xu H, Zhang Y, Liu J, Wang Y, He F, Li Y, Li D (2019) OsteoporosAtlas: a human osteoporosis-related gene database. PeerJ 7:e6778
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods (San Diego, Calif) 25:402–408
Moll JM (1987) New criteria for the diagnosis of ankylosing spondylitis. Scand J Rheumatol Suppl 65:12–24
Xu XM, Li N, Li K, Li XY, Zhang P, Xuan YJ, Cheng XG (2019) Discordance in diagnosis of osteoporosis by quantitative computed tomography and dual-energy X-ray absorptiometry in Chinese elderly men. J Orthop Transl 18:59–64
Kirsten ML, Baron RA, Seabra MC, Ces O (2013) Rab1a and Rab5a preferentially bind to binary lipid compositions with higher stored curvature elastic energy. Mol Membr Biol 30:303–314
Kreivi JP, Trinkle-Mulcahy L, Lyon CE, Morrice NA, Cohen P, Lamond AI (1997) Purification and characterisation of p99, a nuclear modulator of protein phosphatase 1 activity. FEBS Lett 420:57–62
Maruyama K, Uematsu S, Kondo T, Takeuchi O, Martino MM, Kawasaki T, Akira S (2013) Strawberry notch homologue 2 regulates osteoclast fusion by enhancing the expression of DC-STAMP. J Exp Med 210:1947–1960
Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM (2009) A census of human transcription factors: function, expression and evolution. Nat Rev Genet 10:252–263
Bordeleau ME, Aucagne R, Chagraoui J, Girard S, Mayotte N, Bonneil É, Thibault P, Pabst C, Bergeron A, Barabé F, Hébert J, Sauvageau M, Boutonnet C, Meloche S, Sauvageau G (2014) UBAP2L is a novel BMI1-interacting protein essential for hematopoietic stem cell activity. Blood 124:2362–2369
Da Costa R, Bordessoules M, Guilleman M et al (2020) Vps13b is required for acrosome biogenesis through functions in Golgi dynamic and membrane trafficking. Cell Mol Life Sci 77:511–529
Vijayan V, Khandelwal M, Manglani K, Gupta S, Surolia A (2014) Methionine down-regulates TLR4/MyD88/NF-kappaB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br J Pharmacol 171:107–121
Aryal ACS, Miyai K, Izu Y, Hayata T, Notomi T, Noda M, Ezura Y (2015) Nck influences preosteoblastic/osteoblastic migration and bone mass. Proc Natl Acad Sci U S A 112:15432–15437
Gracey E, Yao Y, Green B, Qaiyum Z, Baglaenko Y, Lin A, Anton A, Ayearst R, Yip P, Inman RD (2016) Sexual dimorphism in the Th17 signature of ankylosing spondylitis. Arthritis Rheumatol (Hoboken, NJ) 68:679–689
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35:600–604
Kim HK, Kim MG, Leem KH (2014) Effects of egg yolk-derived peptide on osteogenic gene expression and MAPK activation. Molecules (Basel, Switzerland) 19:12909–12924
Kim DY, Kim GW, Chung SH (2013) Nectandrin A enhances the BMP-induced osteoblastic differentiation and mineralization by activation of p38 MAPK-Smad signaling pathway. Korean J Physiol Pharmacol 17:447–453
Deng FY, Zhao LJ, Pei YF, Sha BY, Liu XG, Yan H, Wang L, Yang TL, Recker RR, Papasian CJ, Deng HW (2010) Genome-wide copy number variation association study suggested VPS13B gene for osteoporosis in Caucasians. Osteoporos Int 21:579–587
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 8197090753), the Application of Clinical Features of Capital City of Science and Technology Commission China BEIJING Special subject (Z181100001718180), and Medical big data and artificial intelligence of PLA General Hospital research project (2019MBD-022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was approved by the Ethics Committee of the Chinese PLA General Hospital and have been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Liu, WB., Tian, FF. et al. Gender-specific SBNO2 and VPS13B as a potential driver of osteoporosis development in male ankylosing spondylitis. Osteoporos Int 32, 311–320 (2021). https://doi.org/10.1007/s00198-020-05593-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05593-9