Abstract
Summary
Transitioning postmenopausal women with osteoporosis from a bisphosphonate to denosumab appears to be safe and more effective at improving BMD than continuing treatment with a bisphosphonate.
Introduction
We conducted a patient-level pooled analysis of four studies to estimate the efficacy and safety of transitioning to denosumab vs. continuing bisphosphonate treatment in postmenopausal women who previously received oral bisphosphonates.
Methods
Patients received 60 mg denosumab once every 6 months or a bisphosphonate (oral alendronate, risedronate, ibandronate, or intravenous zoledronic acid). Endpoints were change from baseline in lumbar spine, total hip, femoral neck, and 1/3 radius BMD at month 12, change from baseline in serum CTX-1 and P1NP, and incidence of adverse events.
Results
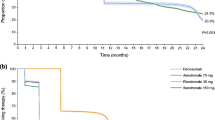
A total of 2850 randomized patients (1424 bisphosphonate:1426 denosumab) were included in the analysis. Percentage change in BMD was significantly greater (p < 0.001) for denosumab vs. bisphosphonate at each skeletal site; differences in BMD changes ranged from 0.6 to 2.0%. Percentage decrease in serum CTX-1 and P1NP was significantly greater (p < 0.0001) for denosumab vs. bisphosphonate at months 1, 6, and 12; in the denosumab group only, percentage change in serum CTX-1 at month 1 was significantly correlated with percentage change in lumbar spine and total hip BMD at month 12. The incidences of adverse events were similar between treatment groups. Three patients (one bisphosphonate and two denosumab) had atypical femoral fractures, all from the denosumab vs. zoledronic acid study.
Conclusion
Postmenopausal women can safely transition from a bisphosphonate to denosumab, which is more effective at improving BMD than continuing with a bisphosphonate.
Clinical trials registration
NCT00377819, NCT00919711, NCT00936897, NCT01732770.



Similar content being viewed by others
References
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, HORIZON Pivotal Fracture Trial (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Byun JH, Jang S, Lee S, Park S, Yoon HK, Yoon BH, Ha YC (2017) The efficacy of bisphosphonates for prevention of osteoporotic fracture: an update meta-analysis. J Bone Metab 24:37–49
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, FREEDOM Trial (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88:2961–2978
Russell RG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119:150–162
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S et al (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145:527–538
Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M et al (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157:435–448
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Yun H, Curtis JR, Guo L, Kilgore M, Muntner P, Saag K, Matthews R, Morrisey M, Wright NC, Becker DJ, Delzell E (2014) Patterns and predictors of osteoporosis medication discontinuation and switching among Medicare beneficiaries. BMC Musculoskelet Disord 15:112
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Boudreau DM, Yu O, Balasubramanian A, Wirtz H, Grauer A, Crittenden DB, Scholes D (2017) A survey of Women’s awareness of and reasons for lack of Postfracture osteoporotic care. J Am Geriatr Soc 65:1829–1835
Gold DT, Martin BC, Frytak JR, Amonkar MM, Cosman F (2007) A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. Curr Med Res Opin 23:585–594
Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E (2011) A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health 14:571–581
Rodan G, Reszka A, Golub E, Rizzoli R (2004) Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin 20:1291–1300
Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna C, Russell RG (2011) The relationship between the chemistry and biological activity of the bisphosphonates. Bone 49:20–33
Lawson MA, Xia Z, Barnett BL, Triffitt JT, Phipps RJ, Dunford JE, Locklin RM, Ebetino FH, Russell RG (2010) Differences between bisphosphonates in binding affinities for hydroxyapatite. J Biomed Mater Res B Appl Biomater 92:149–155
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, Malouf J, Bone HG, Reginster JY, Singer A, Wang C et al (2016) Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab 101:3163–3170
Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, Moffett A, Siddhanti S, Ferreira I, Ghelani P, Wagman RB et al (2013) Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 121:1291–1299
Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola S, Papaioannou N, Stone M, Ferreira I, Siddhanti S, Wagman RB, Brown JP (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58:48–54
Reynolds K, Viswanathan HN, O’Malley CD, Muntner P, Harrison TN, Cheetham TC, Hsu JW, Gold DT, Silverman S, Grauer A, Morisky DE (2012) Psychometric properties of the Osteoporosis-Specific Morisky Medication Adherence Scale in postmenopausal women with osteoporosis newly treated with bisphosphonates. Ann Pharmacother 46:659–670
Reynolds K, Viswanathan HN, Muntner P, Harrison TN, Cheetham TC, Hsu JW, Gold DT, Silverman S, Grauer A, Morisky DE, O’Malley CD (2014) Validation of the Osteoporosis-Specific Morisky Medication Adherence Scale in long-term users of bisphosphonates. Qual Life Res 23:2109–2120
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Howe TS, van der Meulen M, Weinstein RS, Whyte MP (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA 296:2927–2938
Riggs BL, Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20:177–184
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21:1453–1460
Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82:1493–1501
Imel EA, Eckert G, Modi A, Li Z, Martin J, de Papp A, Allen K, Johnston CC, Hui SL, Liu Z (2016) Proportion of osteoporotic women remaining at risk for fracture despite adherence to oral bisphosphonates. Bone 83:267–275
Brown JP (2017) Antiresorptives: safety concerns-clinical perspective. Toxicol Pathol 45:859–863
Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH (2019) Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 104:1753-1765
Wu J, Zhang Q, Yan G, Jin X (2018) Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: a meta-analysis. J Orthop Surg Res 13:194
Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, Marin F, Cauley JA, Bauer DC, Black DM, Project FBQ (2019) Change in Bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 34:632–642
Acknowledgments
Writing support was funded by Amgen Inc. and provided by Kathryn Boorer, PhD, of KB Scientific Communications, LLC.
Funding
This study was funded by Amgen Inc. P.M. has received grants/research support from Amgen, Eli Lilly, Merck, Radius Health, and Ultragenyx and consulting fees from Alexion, Amgen, Eli Lilly, Merck, Radius Health, and Ultragenyx. N.P., A.C., and S.H. are employees and shareholders of Amgen. C.W. was previously employed by Amgen. J.M.-S. has nothing to declare. A.S. has received consulting fees from Agnovos, Amgen, Eli Lilly, Merit, Radius, and UCB; is on the speakers’ bureau for Amgen, Eli Lilly, and Radius; and has a nonremunerative position of influence in the National Osteoporosis Foundation Board of Trustees. E.C. has received grants/research support from Amgen. H.G.B. has received grants/research support from Amgen, Merck, and Shire and consulting fees from Amgen, Grünenthal, Radius Health, and Merck, and is on the speakers’ bureau for Amgen, Radius Health, and Shire. W.L. has received consulting fees from and is on the speakers’ bureau for Amgen, Eli Lilly, Merck, UCB, and Curaphar. J.P.B. has received grants/research support from Amgen and Eli Lilly and consulting fees from Amgen, Eli Lilly, and Merck, and is on the speakers’ bureau for Amgen and Eli Lilly.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Informed consent
Informed consent was obtained from all individual participants included in each study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data sharing statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 177 kb)
Rights and permissions
About this article
Cite this article
Miller, P., Pannacciulli, N., Malouf-Sierra, J. et al. Efficacy and safety of denosumab vs. bisphosphonates in postmenopausal women previously treated with oral bisphosphonates. Osteoporos Int 31, 181–191 (2020). https://doi.org/10.1007/s00198-019-05233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05233-x