Abstract
Summary
This study aimed to investigate the bone impairment in finger joints in PHO patients by HR-pQCT. Results showed distinguished differences in bone architecture and biomechanics parameters at DIPs between PHO patients and healthy controls using HR-pQCT assessment. Besides, serum PGE2, hsCRP and ESR levels were found negatively correlated with total vBMD.
Introduction
This study aimed to investigate the bone impairment in finger joints in primary hypertrophic osteoarthropathy (PHO) patients firstly by high-resolution peripheral quantitative computed tomography (HR-pQCT).
Methods
Fifteen PHO patients and 15 healthy controls were enrolled in this study. Bone erosions in hands at distal interphalangeal joints (DIPs) in both PHO patients and controls were evaluated by X-ray. Bone geometry, vBMD, microstructure parameters, and size of individual bone erosion were also measured at the 3rd DIP by HR-pQCT as well. Blood biochemistry levels between the two groups were also compared.
Results
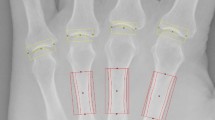
Compared to X-ray, HR-pQCT assessment were more sensitive for detection of bone erosions, with 14 PHO patients by HR-pQCT versus ten PHO patients by X-ray judged at the 3rd DIP. The average depth, width, and volume of erosions size in PHO patients were 1.38 ± 0.80 mm, 0.79 ± 0.27 mm, and 1.71 ± 0.52 mm3, respectively. The bone cross-areas including total area (+ 25.3%, p ≤ 0.05), trabecular area (+ 56.2%, p ≤ 0.05), and cortical perimeter (+ 10.7%, p ≤ 0.05) at the defined region of interest of 3rd DIP was significantly larger than controls. Total vBMD was 11.9% lower in PHO patients compared with the controls (p ≤ 0.05). Biochemical test results showed the increased levels of inflammatory cytokines, bone resorption markers, and joint degeneration markers in PHO patients. Serum prostaglandin PGE2, high-sensitive C-reactive protein (hsCRP) and erythrocyte sedimentation rate (ESR) levels were found negatively correlated with total vBMD.
Conclusions
This study demonstrated higher sensitivity of the HR-pQCT measurement at DIPs by showing the differences in architecture and biomechanics parameters at DIPs between the PHO patients and healthy controls, which would be of interest clinically to investigate bone deterioration in PHO patients.

Similar content being viewed by others
References
Zhang Z, Zhang C, Zhang Z (2013) Primary hypertrophic osteoarthropathy: an update. Front Med 7:60–64
Uppal S, Diggle CP, Carr IM, Fishwick CW, Ahmed M, Ibrahim GH, Helliwell PS, Latos-Bielenska A, Phillips SE, Markham AF, Bennett CP, Bonthron DT (2008) Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet 40:789–793
Zhang Z, Xia W, He J, Zhang Z, Ke Y, Yue H, Wang C, Zhang H, Gu J, Hu W, Fu W, Hu Y, Li M, Liu Y (2012) Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet 90:125–132
Li SS, He JW, Fu WZ, Liu YJ, Hu YQ, Zhang ZL (2017) Clinical, biochemical, and genetic features of 41 Han Chinese families with primary hypertrophic osteoarthropathy, and their therapeutic response to etoricoxib: results from a six-month prospective clinical intervention. J Bone Miner Res 32:1659–1666
Yap FY, Skalski MR, Patel DB, Schein AJ, White EA, Tomasian A, Masih S, Matcuk GJ (2017) Hypertrophic osteoarthropathy: clinical and imaging features. Radiographics 37:157–195
Diggle CP, Carr IM, Zitt E, Wusik K, Hopkin RJ, Prada CE, Calabrese O, Rittinger O, Punaro MG, Markham AF, Bonthron DT (2010) Common and recurrent HPGD mutations in Caucasian individuals with primary hypertrophic osteoarthropathy. Rheumatology 49(6):1056–1062
Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, Im HJ (2009) Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum 60:513–523
McCoy JM, Wicks JR, Audoly LP (2002) The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest 110:651–658
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000
Hou Y, Lin Y, Qi X, Yuan L, Liao R, Pang Q, Cui L, Jiang Y, Wang O, Li M, Dong J, Xia W (2018) Identification of mutations in the prostaglandin transporter gene SLCO2A1 and phenotypic comparison between two subtypes of primary hypertrophic osteoarthropathy (PHO): A single-center study. Bone 106:96–102
Rendina D, De Filippo G, Viceconti R, Soscia E, Sirignano C, Salvatore M, Della MM, Scarano G, Mossetti G (2008) Interleukin (IL)-6 and receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) are increased in the serum of a patient with primary pachydermoperiostosis. Scand J Rheumatol 37:225–229
Adams B, Amin T, Leone V, Wood M, Kraft JK (2016) Primary hypertrophic osteoarthropathy: ultrasound and MRI findings. Pediatr Radiol 46:727–730
Redlich K, Smolen JS (2012) Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 11:234–250
Ejbjerg B, Narvestad E, Rostrup E, Szkudlarek M, Jacobsen S, Thomsen HS, Ostergaard M (2004) Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum 50:1097–1106
Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, van den Bergh J (2014) High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol 10:304–313
Dohn UM, Ejbjerg BJ, Hasselquist M, Narvestad E, Moller J, Thomsen HS, Ostergaard M (2008) Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res Ther 10:R25
Regensburger A, Rech J, Englbrecht M, Finzel S, Kraus S, Hecht K, Kleyer A, Haschka J, Hueber AJ, Cavallaro A, Schett G, Faustini F (2015) A comparative analysis of magnetic resonance imaging and high-resolution peripheral quantitative computed tomography of the hand for the detection of erosion repair in rheumatoid arthritis. Rheumatology (Oxford) 54:1573–1581
Stach CM, Bauerle M, Englbrecht M, Kronke G, Engelke K, Manger B, Schett G (2010) Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high-resolution computed tomography. Arthritis Rheum 62:330–339
Fouque-Aubert A, Boutroy S, Marotte H, Vilayphiou N, Bacchetta J, Miossec P, Delmas PD, Chapurlat RD (2010) Assessment of hand bone loss in rheumatoid arthritis by high-resolution peripheral quantitative CT. Ann Rheum Dis 69:1671–1676
Altman RD, Gold GE (2007) Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 15(Suppl A):A1–A56
Srikhum W, Virayavanich W, Burghardt AJ, Yu A, Link TM, Imboden JB, Li X (2013) Quantitative and semiquantitative bone erosion assessment on high-resolution peripheral quantitative computed tomography in rheumatoid arthritis. J Rheumatol 40:408–416
Finzel S, Ohrndorf S, Englbrecht M, Stach C, Messerschmidt J, Schett G, Backhaus M (2011) A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum 63:1231–1236
Yue J, Griffith JF, Xiao F, Shi L, Wang D, Shen J, Wong P, Li EK, Li M, Li TK, Zhu TY, Hung VW, Qin L, Tam LS (2017) Repair of bone erosion in rheumatoid arthritis by denosumab: a high-resolution peripheral quantitative computed tomography study. Arthritis Care Res 69:1156–1163
Tang XL, Qin L, Kwok AW, Zhu TY, Kun EW, Hung VW, Griffith JF, Leung PC, Li EK, Tam LS (2013) Alterations of bone geometry, density, microarchitecture, and biomechanical properties in systemic lupus erythematosus on long-term glucocorticoid: a case-control study using HR-pQCT. Osteoporos Int 24:1817–1826
Ding M, Lin XZ, Liu WG (2018) Three-dimensional morphometric properties of rod- and plate-like trabeculae in adolescent cancellous bone. J Orthop Translat 12:26–35
Zhu TY, Griffith JF, Qin L, Hung VW, Fong TN, Au SK, Tang XL, Kwok AW, Leung PC, Li EK, Tam LS (2013) Structure and strength of the distal radius in female patients with rheumatoid arthritis: a case-control study. J Bone Miner Res 28:794–806
Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL (2013) Mutations in the SLCO2A1 gene and primary hypertrophic osteoarthropathy: a clinical and biochemical characterization. J Clin Endocrinol Metab 98:E923–E933
Yuan L, Chen L, Liao RX, Lin YY, Jiang Y, Wang O, Li M, Xing XP, Pang QQ, Jiajue R, Xia WB (2015) A common mutation and a novel mutation in the HPGD gene in nine patients with primary hypertrophic osteoarthropathy. Calcif Tissue Int 97:336–342
Erken E, Koroglu C, Yildiz F, Ozer HT, Gulek B, Tolun A (2015) A novel recessive 15-hydroxyprostaglandin dehydrogenase mutation in a family with primary hypertrophic osteoarthropathy. Mod Rheumatol 25:315–321
Madruga DJ, Rosa RS, Perpetuo I, Rodrigues AM, Janeiro A, Costa MM, Gaiao L, Pereira DSJ, Fonseca JE, Miltenberger-Miltenyi G (2014) Pachydermoperiostosis in an African patient caused by a Chinese/Japanese SLCO2A1 mutation-case report and review of literature. Semin Arthritis Rheum 43:566–569
Seifert W, Kuhnisch J, Tuysuz B, Specker C, Brouwers A, Horn D (2012) Mutations in the prostaglandin transporter encoding gene SLCO2A1 cause primary hypertrophic osteoarthropathy and isolated digital clubbing. Hum Mutat 33:660–664
Kwok WY, Kloppenburg M, Rosendaal FR, van Meurs JB, Hofman A, Bierma-Zeinstra SM (2011) Erosive hand osteoarthritis: its prevalence and clinical impact in the general population and symptomatic hand osteoarthritis. Ann Rheum Dis 70:1238–1242
Peters M, van Tubergen A, Scharmga A, Driessen A, van Rietbergen B, Loeffen D, Weijers R, Geusens P, van den Bergh J (2018) Assessment of cortical interruptions in the finger joints of patients with rheumatoid arthritis using HR-pQCT, radiography, and MRI. J Bone Miner Res 33:1676–1685
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8:656–664
Gruneboom A, Hawwari I, Weidner D, Culemann S, Muller S, Henneberg S, Brenzel A, Merz S, Bornemann L, Zec K, Wuelling M, Kling L, Hasenberg M, Voortmann S, Lang S, Baum W, Ohs A, Kraff O, Quick HH, Jager M, Landgraeber S, Dudda M, Danuser R, Stein JV, Rohde M, Gelse K, Garbe A, Adamczyk A, Westendorf AM, Hoffmann D, Christiansen S, Engel DR, Vortkamp A, Kronke G, Herrmann M, Kamradt T, Schett G, Hasenberg A, Gunzer M (2019) A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab 1:236–250
Scharmga A, Keller KK, Peters M, van Tubergen A, van den Bergh JP, van Rietbergen B, Weijers R, Loeffen D, Hauge EM, Geusens P (2017) Vascular channels in metacarpophalangeal joints: a comparative histologic and high-resolution imaging study. Sci Rep 7:8966
Siu WS, Qin L, Leung KS (2003) pQCT bone strength index may serve as a better predictor than bone mineral density for long bone breaking strength. J Bone Miner Metab 21:316–322
Greene D, Naughton G, Briody J, Kemp A, Woodhead H, Corrigan L, Karisson M (2005) Bone strength index in adolescent girls: does physical activity make a difference? Br J Sports Med 39:622–627
Ke HZ, Jee WS, Mori S, Li XJ, Kimmel DB (1992) Effects of long-term daily administration of prostaglandin-E2 on maintaining elevated proximal tibial metaphyseal cancellous bone mass in male rats. Calcif Tissue Int 50:245–252
Turner CH, Forwood MR, Rho JY, Yoshikawa T (1994) Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res 9:87–97
Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ (2002) Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest 109:1405–1415
Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Ronday HK, Seys PE, Kerstens PJ, Huizinga TW, Dijkmans BA, Allaart CF (2010) A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 69:1333–1337
Finzel S, Rech J, Schmidt S, Engelke K, Englbrecht M, Stach C, Schett G (2011) Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann Rheum Dis 70:1587–1593
Devlin J, Lilley J, Gough A, Huissoon A, Holder R, Reece R, Perkins P, Emery P (1996) Clinical associations of dual-energy X-ray absorptiometry measurement of hand bone mass in rheumatoid arthritis. Br J Rheumatol 35:1256–1262
Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I (2000) Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27:351–357
Acknowledgments
We would like to give the sincere thanks to the subjects for consenting to participate in this study.
Funding
This study was supported by the National Natural Science Foundation of China (No.81471088 and No. 81670714) and Bone Quality and Health Assessment Center of the Department of Orthopaedics and Traumatology, the Chinese University of Hong Kong for providing hand fixator for HR-pQCT scanning.
Author information
Authors and Affiliations
Contributions
Prof. Ling Qin and Prof. Weibo Xia were responsible for study design. Ms. Qianqian Pang performed the study and prepared the first draft of the paper. Ms. Qianqian Pang, Dr. Ruoxi Liao, Ms. Yuping Xu, Ms. Yanfang Hou, and Dr. Jiankun Xu contributed to the clinical and experimental work. Dr. Vivian W Hung, Dr. Xuan Qi, and Ms. Qianqian Pang contributed to the imaging analyses. Ms. Qianqian Pang, Dr. Xuan Qi, and Dr. Le Huang were responsible for statistical analysis of the data. All of the authors contributed to revise the paper critically for intellectual content and approved the final version of the submitted manuscript.
Corresponding authors
Ethics declarations
All subjects signed informed consent for imaging measures as well as blood sample collections. This study was approved by the Ethics Committee of PUMCH with the ethics audit number zs-1115.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pang, Q., Xu, Y., Qi, X. et al. Impaired bone microarchitecture in distal interphalangeal joints in patients with primary hypertrophic osteoarthropathy assessed by high-resolution peripheral quantitative computed tomography. Osteoporos Int 31, 153–164 (2020). https://doi.org/10.1007/s00198-019-05168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05168-3