Abstract
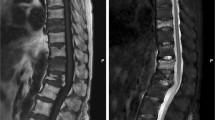
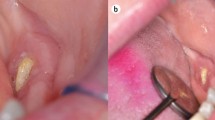
We reported a 69-year-old female who discontinued denosumab due to dental treatment and subsequently suffered rebound-associated vertebral fractures 10 months after the last injection. This case raised an alarm regarding the discontinuation of denosumab for dental treatment. Denosumab, a human monoclonal antibody administered by subcutaneous injection, to the best of our knowledge, is the only fully investigated inhibitor of receptor activator of nuclear factor kappa B ligand. Discontinuation of denosumab leads to bone turnover rebound and rapid bone mineral density loss. Several studies have reported rebound-associated vertebral fractures after discontinuation of denosumab. We report on a new case of rebound-associated vertebral fractures after discontinuation of denosumab. A 69-year-old female, who withdrew from denosumab treatment after 3 years due to maxillitis, presented to our hospital with severe low back pain without any history of trauma. Ten months had passed since the last injection. Magnetic resonance imaging showed five acute vertebral fractures, which appeared to be rebound-associated vertebral fractures caused by discontinuation of denosumab due to dental treatment. This case clearly demonstrates the risk of discontinuation of denosumab for dental treatment.


Similar content being viewed by others
References
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Ström O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411. https://doi.org/10.1007/s00198-015-3253-4
Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, Brandi ML, Czerwiński E, Franek E, Lakatos P, Mautalen C, Minisola S, Reginster JY, Jensen S, Daizadeh NS, Wang A, Gavin M, Libanati C, Wagman RB, Bone HG (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM extension study. Osteoporos Int 26(12):2773–2783. https://doi.org/10.1007/s00198-015-3234-7
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96(4):972–980. https://doi.org/10.1210/jc.2010-1502
O’Halloran M, Boyd NM, Smith A (2014) Denosumab and osteonecrosis of the jaws—the pharmacology, pathogenesis and a report of two cases. Aust Dent J 59(4):516–519. https://doi.org/10.1111/adj.12217
Khan A, Morrison A, Cheung A, Hashem W, Compston J (2016) Osteonecrosis of the jaw (ONJ): diagnosis and management in 2015. Osteoporos Int 27(3):853–859. https://doi.org/10.1007/s00198-015-3335-3
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res 32(6):1291–1296. https://doi.org/10.1002/jbmr.3110
Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B (2017) Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 102(2):354–358. https://doi.org/10.1210/jc.2016-3170
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27(5):1923–1925. https://doi.org/10.1007/s00198-015-3380-y
Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A (2014) Determinants associated with bone mineral density increase in response to daily teriparatide treatment in patients with osteoporosis. Bone 66:26–30. https://doi.org/10.1016/j.bone.2014.05.017
Brown JP, Roux C, Törring O, Ho PR, Beck Jensen JE, Gilchrist N, Recknor C, Austin M, Wang A, Grauer A, Wagman RB (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the fracture reduction evaluation of Denosumab in osteoporosis every 6 months (FREEDOM) trial. J Bone Miner Res 28(4):746–752. https://doi.org/10.1002/jbmr.1808
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Beck Jensen JE, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP (2017) Vertebral fractures following discontinuation of denosumab: a post-hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28(5):1723–1732. https://doi.org/10.1007/s00198-017-3919-1
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL, Investigators DAPS (2012) Final results of the DAPS (Denosumab adherence preference satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23(1):317–326. https://doi.org/10.1007/s00198-011-1780-1
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-switch study): extension of a randomised controlled trial. Lancet 386(9999):1147–1155
McClung MR (2016) Cancel the denosumab holiday. Osteoporos Int 27(5):1677–1682. https://doi.org/10.1007/s00198-016-3553-3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Rights and permissions
About this article
Cite this article
Niimi, R., Kono, T., Nishihara, A. et al. Rebound-associated vertebral fractures after discontinuation of denosumab for the treatment of maxillitis. Osteoporos Int 29, 769–772 (2018). https://doi.org/10.1007/s00198-017-4334-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4334-3