Abstract
Summary
Osteoporosis represents a significant and increasing healthcare burden in Europe, but most patients at increased risk of fracture do not receive medication, resulting in a large treatment gap. Identification of patients who are at particularly high risk will help clinicians target appropriate treatment more precisely and cost-effectively, and should be the focus of future research.
Introduction
The purpose of the study was to review data on the identification and treatment of patients with osteoporosis at increased risk of fracture.
Methods
A working group convened by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis met to review current data on the epidemiology and burden of osteoporosis and the patterns of medical management throughout Europe.
Results
In Europe in 2010, the cost of managing osteoporosis was estimated at €37 billion and notably the costs of treatment and long-term care of patients with fractures were considerably higher than the costs for pharmacological prevention. Despite the availability of effective treatments, the uptake of osteoporosis therapy is low and declining, in particular for secondary fracture prevention where the risk of a subsequent fracture following a first fracture is high. Consequently, there is a significant treatment gap between those who would benefit from treatment and those who receive it, which urgently needs to be addressed so that the burden of disease can be reduced.
Conclusions
Implementation of global fracture prevention strategies is a critical need. Future research should focus on identifying specific risk factors for imminent fractures, periods of high fracture risk, patients who are at increased risk of fracture and therapies that are most suited to such high-risk patients and optimal implementation strategies in primary, secondary and tertiary care.
Similar content being viewed by others
Introduction
Osteoporosis represents a substantial and increasing burden on healthcare systems in many countries around the world. The resulting fractures are associated with reduced quality of life and significant morbidity, mortality and healthcare resource utilisation [1]. However, most patients who have sustained a fracture or who are at increased risk of fracture do not receive appropriate osteoporosis treatment, and treatment rates have declined in recent years [1]. This increasing ‘treatment gap’ suggests the need for both better evaluation of patients and consensus among clinicians regarding the definition of those at increased risk of fracture and how they should best be treated. To address this, a working group convened by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) met to review current data on the epidemiology and burden of osteoporosis and the patterns of medical management throughout Europe. This manuscript summarises the working group’s views and recommendations regarding strategies for the identification of patients at increased risk of fracture, the currently available options for their effective management and data for new products in development.
Methods
As in previous initiatives and publications [2], the ESCEO working group consisted of clinical scientists and experts in the field of osteoporosis. Different members of the ESCEO working group were asked to prepare a full review of the literature on the following: (a) Epidemiology of spinal and non-spinal fractures in Europe (CC); (b) Burden of spinal and non-spinal fractures in Europe (MLB); (c) Management of osteoporosis in Europe—the treatment gap (JAK); (d) How can we define a patient at high (imminent) risk of fracture (NCH); and (e) Efficacy of currently available treatments in patients at high risk of fracture (TT). Each member prepared a list of the most important topics based on their review of the literature and then made a set of preliminary recommendations. The subsequent step was a face-to-face meeting for the whole group (9 September 2016) to make amendments and discuss further recommendations. The plan of the manuscript was also discussed, and shared conclusions were reached. The present recommendations were developed independently of any of the funding sources that had no role in the decision to prepare this document and its implementation, revisions and approval for publication. In addition, each member of the task force individually agreed to declare their potential conflict of interest, if any, in the process of article submission.
Epidemiology of vertebral and non-vertebral fractures in Europe
More than 8.9 million osteoporotic fractures occur annually worldwide, and approximately one third of those fractures are in Europe, equating to 3.5 million cases per year [1]. A review of the clinical and economic burden of osteoporotic fractures in 27 European countries in 2010 found that two thirds of all incident fractures occurred in women and fracture incidence increased with age, with the majority of hip fractures reported in patients aged ≥80 years [1]. The most common fractures were hip (18%), forearm (16%), vertebral (15%) and ‘others’ (51%) (Fig. 1) [1]. Other studies, such as the POSSIBLE EU® study, have also highlighted the importance of non-hip and non-vertebral (NHNV) fractures, where 70% of fractures occurred in NHNV locations in postmenopausal women receiving bone loss therapies in a primary care setting [3]. Historically, the importance of vertebral fracture incidence has been relatively inflated due to its use as a primary endpoint in many clinical studies, where too much emphasis has been placed on the importance of grade 1 vertebral fractures. While such vertebral deformities may have some prognostic value for further vertebral fractures, they have little or no prognostic value for non-vertebral fractures; therefore, more focus should be placed on grade 2 and 3 fractures [4]. With the increasing prominence of non-vertebral fracture incidence as a discriminator between investigational drugs in more recent clinical studies, a balance between the relative significances of different fracture types is needed.
Estimated number of incident fractures by type and country in the European Union in 2010 [1]
Geographically, fracture incidence varies widely by country across Europe (Fig. 1) [1, 5]. Compared with other regions of the world, Europe has some of the highest hip fracture rates, with an apparent north–south gradient, and most countries are categorised as high or moderate risk [5]. However, this variation between countries is not as pronounced for vertebral fracture incidence [6], at least when judged by vertebral morphometry. The underlying causes of these variations are unknown but are likely to be environmental rather than genetic [5]. Socioeconomic factors have been hypothesised as being the most likely explanation for the heterogeneity of fracture incidence between communities, but other factors are also candidates, such as sunlight exposure, low calcium intake, physical activity, low body mass index, anthropometric variables and race [1, 7, 8].
Substantial temporal trends in age-specific rates of hip fracture have been observed in recent decades. With a few exceptions, age-specific incidence rates rose in western populations until around 1980 and have since either reached a plateau or declined [6]. In the case of hip fracture incidence rates, an earlier reversal of this trend along with a higher peak fracture incidence has been demonstrated in northern European countries compared with a later trend for reversal and lower peak fracture incidence in some southern European countries [9]. A recent study of hip fracture trends in Sweden and Denmark found that period and cohort effects, which may reflect environmental and lifestyle factors, contributed to this observation, and analyses indicated that age-specific hip fracture rates were likely to increase again in the near future [10].
Further to this, the total number of people with osteoporosis in Europe has been predicted to rise by 23%, from 27.5 million in 2010 to 33.9 million in 2025 due to the increasing proportion of elderly people in the population. As a consequence, the osteoporotic fracture rate is also expected to increase throughout Europe, with an increase of 56 and 41% predicted in the male and female populations, respectively [1].
Burden of vertebral and non-vertebral fractures in Europe
Hip and vertebral fractures are associated with increased mortality, with the mortality risk highest immediately after the fracture event and then decreasing with time [11]. In Europe, the number of deaths in 2010 directly related to fractures was estimated at approximately 20,100 in men and 22,700 in women, of which 49 and 33% were attributed to hip and vertebral fracture events, respectively [1].
The management of osteoporosis is also associated with a very high economic burden in Europe, with a high degree of variation between countries (Fig. 2). In 2010, the cost of managing osteoporosis was estimated at €37 billion. Despite this, there is currently minimal investment in pharmacological prevention, which comprised 5% of this cost, compared with the costs of treating incident fractures (66%) and long-term fracture care (29%) [1]. As a proportion of the total spend, excluding expenditure for pharmacological prevention, hip fractures represented 54%, while ‘other fractures’ represented 39%, and clinical vertebral and forearm fractures only represented 5 and 2%, respectively [1]. The significant impact of NHNV fractures in particular on costs and healthcare resources has also been demonstrated in other studies, such as the Global Longitudinal Study of Osteoporosis in Women (GLOW) study where NHNV fractures resulted in a substantially higher number of days in hospital and rehabilitation/nursing home care over a 1-year period compared with vertebral and hip fractures [13].
When considering quality-adjusted life-years (QALYs), which give a societal perspective on the burden of disease, the total health burden of osteoporosis in Europe in 2010 was estimated at 1,165,000 QALYs, and twice as many QALYs were lost in women compared with men [1]. Hip fractures, clinical vertebral, forearm and other fractures incurred approximately 600,000 (52%), 344,000 (30%), 19,000 (2%) and 202,000 (17%) QALYs lost, respectively. For hip and vertebral fractures, approximately 79 and 59% of the QALYs lost were a consequence of prior fractures [1]. When the cost of osteoporosis was combined with the value for QALYs lost, the overall cost of osteoporosis amounted to €98 billion in Europe in 2010.
This burden associated with osteoporosis has been shown to be higher than for other common non-communicable diseases. Total disability-adjusted life-years (DALYs) lost due to osteoporosis in Europe, reflecting the years of life lost due to a fracture and the disability in those who survive, were 5.8 million in 2010, representing 0.83% of the global burden of non-communicable disease. This loss in DALYs for osteoporosis was greater than for other diseases such as hypertensive heart disease and rheumatoid arthritis [1]. Furthermore, fractures due to osteoporosis accounted for more deaths and morbidity than any cancer type other than lung cancer [1].
The already high healthcare costs of osteoporosis in Europe are predicted to increase in the future due to the growing elderly population. The annual number of QALYs lost annually in Europe is expected to rise, such that by 2025, it will have increased by 20% from 2010, with the highest growth (32%) forecast for the population aged ≥80 years, who incur the highest costs for fractures compared with other age groups [1]. Overall, the total cost (including values of QALYs lost) in Europe is predicted to rise by 23%, from €98 billion in 2010 to €120 billion in 2025.
It is apparent that the very high costs associated with osteoporosis and its management in Europe, coupled with the predicted future cost increases, highlight the critical need for a change in healthcare policy and the importance of implementing preventative strategies to reduce this high burden of disease.
Management of osteoporosis in Europe: the treatment gap
Approximately 6.8 million men and women in Europe had sustained a prior hip or clinical vertebral fracture in 2010 [1]. It is well known that the risk of a subsequent fracture increases significantly following a first fracture [14–17], yet despite this and the advances in osteoporosis treatment, patients with a prior fracture are reported to have a low uptake of treatments for secondary prevention of fracture and indeed, worldwide [1, 18–23]. Prospective and observational studies, including data from the GLOW study, suggest that only 20% of eligible patients receive osteoporosis treatment after fracture, although uptake varies widely by country in Europe [1, 18]. Of great concern is that treatment uptake has also been shown to be decreasing over time [1]. In a retrospective, observational cohort study in the USA, the estimated probability of osteoporosis medication use in the year after hip fracture declined significantly from 40 to 21% over the 10-year study period [19]. Treatment use was also lower in older patients than younger patients [19], demonstrating that those who needed treatment the most are maybe the least likely to receive it.
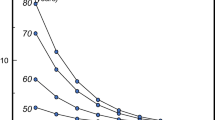
In Europe, there was a general trend towards an increase in treatment uptake until 2006–2008, after which there was a plateau and subsequent decrease in many countries up to 2010–2012, most markedly for bisphosphonates; non-bisphosphonate use had a continuing modest increase [1, 24] (Fig. 3). A similar trend has been observed in the USA [25]. Uptake of osteoporosis treatments varies substantially between countries in Europe, generally being lower than average in northern and eastern Europe (with the exception of Ireland and Hungary), while western Europe has the highest coverage [1]. Added to this is the influence of patient adherence to treatment, as demonstrated in a Swedish study where approximately 50% of all treatment-naïve patients discontinued treatment for osteoporosis within 1 year [26].
Estimated sales (defined daily doses [DDDs]/100 population aged 50+ years) from 2001 to 2011 [1]. Alendronate, etidronate, risedronate and raloxifene were available before 2001. PTH parathyroid hormone. Reprinted with kind permission from Springer Science and Business Media
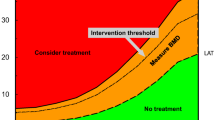
Various approaches, using for example prescription claims data, can be used to characterise the treatment gap: the proportion of patients treated in relation to those eligible for treatment based on their fracture risk. The probability of fracture can be assessed using FRAX, which integrates the weight of clinical risk factors for fracture, with or without information on bone mineral density (BMD), to give the 10-year probability of hip fracture or a major osteoporotic fracture (MOF) (clinical spine, hip, forearm or humerus) [27]. As FRAX country models are calibrated for fracture incidence, there is a large expected heterogeneity between countries in the probability of fracture [1, 5]. Furthermore, an intervention threshold must be defined to be able to characterise the treatment gap, e.g. the FRAX-based UK National Osteoporosis Guidelines Group intervention threshold of a 10-year fracture probability equivalent to women with a prior fragility fracture without knowledge of BMD [27], which is increasingly being adopted in country-specific assessment guidelines [28].
Using FRAX to determine the 10-year probability of a MOF for women at the fracture threshold, a significant treatment gap has been identified in Europe (Fig. 4), with only 59% of men and 57% of women receiving treatment out of the population considered eligible for treatment [1]. A large degree of heterogeneity is evident between countries, with the treatment gap for women ranging from 95% in Bulgaria to 25% in Spain. There are multiple reasons underlying this large treatment gap, such as clinicians not adhering to treatment guidelines and reimbursement issues. Another contributor is poor patient adherence to treatment, which may be influenced by the fact that older patients often have comorbidities and need to take multiple medications. Also, some treatments have only moderate efficacy; studies of goal-directed treatment in osteoporosis have highlighted difficulties in meeting treatment goals with existing therapies in high-risk patients [29]. Furthermore, some treatments require frequent dosing or are associated with side effects, which can result in patients being reluctant to persist with treatment [27]. This has been illustrated in recent years by the overly negative reaction of the medical community and lay press to reports of rare side effects of bisphosphonates, such as osteonecrosis of the jaw, atrial fibrillation, atypical femur fracture and oesophageal cancer, which have concerned patients and discouraged general practitioners from prescribing bisphosphonates, therefore impacting on uptake of these treatments [30]. Reports of osteonecrosis of the jaw with bisphosphonates have also resulted in an overreaction from the dental community. Consequently, and in contrast to that observed for generic medications in other diseases (i.e. statins for hypercholesterolaemia), the availability of generic bisphosphonates has had little impact on treatment uptake [1, 25]. Furthermore, general practitioners may still perceive therapies for osteoporosis as being too expensive. Another reason for the treatment gap may be that, unlike some other chronic diseases such as diabetes, patients do not see an immediate change in their condition and some may not understand parameters used to monitor their condition, such as biomarkers or BMD. Comparisons between the efficacy of osteoporosis drugs in preventing poor outcomes and mortality and the efficacy of treatments for other chronic diseases should be emphasised in order to correct the misperception that osteoporosis is not a serious disease and that osteoporosis drugs are similarly effective as those in other conditions.
Strategies are urgently needed to be implemented to close the treatment gap. Firstly, as they are recognised to be at greater risk of a second fracture, patients with a prior fracture should be assessed and managed appropriately. Screening programmes to identify patients at increased risk of fracture are currently being evaluated for their effectiveness in reducing fracture incidence. The SCOOP study in the UK was a seven-centre, randomised, controlled study with 5-year follow-up that assessed the effectiveness of a community-based screening programme using FRAX to assess women at high risk of hip fracture [31]. The study confirmed the feasibility of screening in the UK, and while investigators did not observe an overall reduction in fracture rates or mortality, hip fracture rates were significantly reduced by nearly 30%.
Further education is also needed for both healthcare professionals and patients to encourage adherence to publish treatment guidelines and to put into context the perceived versus actual risks of reported bisphosphonates side effects. Clearer communication to patients regarding monitoring methods will also be of value so that they understand the effects of their treatment and importance of adherence.
Finally, efforts to close the treatment gap may be further enhanced by implementing fracture liaison services, which have been shown to improve uptake of osteoporosis intervention guidelines, reduce re-fracture rates and increase the cost-effectiveness of treatment [22, 32–34]. Global initiatives, such as the International Osteoporosis Foundation’s (IOF) ‘Capture the Fracture®’ campaign, aim to develop a best practice framework for fracture liaison services and provide support and resources that facilitate their implementation at a local level. Furthermore, the clinical community in Europe needs to learn from healthcare systems in countries, where the treatment gap is low, to develop a more consistent approach to osteoporosis care.
How can we define a patient at increased risk of fracture?
First, we should consider what we mean by an increased risk of fracture and what time scale would be appropriate for this metric. Were we able to deliver entirely safe and cost-effective interventions with high adherence, then lifelong interventions might be envisaged. Given that this is not (yet) possible, then targeting treatment to those at high risk becomes an inevitability. Compared with the young, the elderly are at greatly increased risk of fracture [35] and women are at increased risk compared with men [1]. The risk of fracture is also higher in those with low BMD and in those who are prone to falling or have sustained prior low-energy fractures [1, 15, 36]. As the efficacy of pharmaceutical intervention for osteoporosis has been tested in, at best, studies of 5 or 10 years’ duration, these are more intuitive time scales over which to address fracture risk than, for example, lifetime risk of fracture.
Whereas there are many possible predictors of incident fracture, the time course of the relationship of these risk factors with fracture is currently still uncertain. What we do know is that previous fracture at different sites is a well-documented risk factor for future fracture, and this risk is highest immediately after the initial event and subsequently declines with time [14–17] (Fig. 5). This has recently been demonstrated in the Reykjavik study, where the risk of a MOF after a first MOF was 2.7-fold higher compared with the population risk at 1 year, decreasing to 1.4-fold after 10 years [14]; this higher risk of a second MOF increased by 4% for each year of age. Furthermore, prior fractures continue to be an important predictor of fracture risk for up to 10 years, even in models adjusted for age, BMD and other FRAX clinical risk factors [37]. Further studies are needed to analyse the determinants of imminent risk, e.g. whether the type of fracture affects the future risk, and whether the risks identified are responsive to medical intervention.
Risk per 100,000 (95% confidence interval) of a second major osteoporotic fracture (MOF) after a first MOF for a woman aged 75 years at her first fracture. Knots for the spline function are set at 0.5, 2.5 and 15 years of follow-up after the first fracture. The dashed line is the risk of first MOF in the whole population (n = 18,872) for a woman aged 75 years at baseline [14]. Reprinted with kind permission from Springer Science and Business Media
Efficacy of currently available treatments in patients at increased risk of fracture
A range of therapies are approved, with others under investigation for the prevention and treatment of osteoporosis [27]. They are broadly divided into two categories: inhibitors of bone resorption by osteoclasts (including bisphosphonates, selective oestrogen receptor modulators and monoclonal antibodies to the receptor activator of nuclear factor kappa-B ligand) and anabolic agents that stimulate bone formation, such as teriparatide (parathyroid hormone [PTH] 1–34). Strontium ranelate is another agent that reduces fracture risk. The mechanism of action of strontium ranelate remains unclear, but it appears to have weak effects on bone turnover and changes in bone quality. In the clinical study setting, different approaches are used to evaluate the efficacy of these approved and investigational treatments in patients at high risk of fracture, including the selection of such patients using specific inclusion criteria, subgroup analysis using risk factors known to further increase fracture risk or analyses using a continuous variable of fracture probability, such as FRAX.
Differences in clinical study inclusion criteria, as well as baseline disease severity, can result in challenges when evaluating the optimal treatment for patients at increased risk of fracture [38]. For example, the phase 3, double-blind, randomised, placebo-controlled Abalopartide comparator trial in vertebral endpoints (ACTIVE) study evaluating the investigational drug abaloparatide recruited patients at low risk of fracture [39] compared with others, such as the TROPOS and SOTI studies [40, 41], which evaluated the safety and efficacy of strontium ranelate in patients with baseline characteristics that were more indicative of a higher risk population. These two examples highlight the discordances that can arise because of variable inclusion criteria, resulting in different population characteristics, which can make data comparison and interpretation difficult.
Broad inclusion criteria in positive clinical studies enable analyses to be performed to investigate interactions between baseline risk factors, such as FRAX, BMD, age or prior fractures and treatment response. These analyses require a sufficiently large number of high-risk patients to provide a meaningful understanding of the effect of treatment in such patients. Some subgroup analyses from clinical studies have shown at least comparable efficacy in patients at increased risk of fracture compared with the overall population. For example, analyses of the effect of baseline age, vertebral BMD and prevalent vertebral fractures on the therapeutic effect of abaloparatide in postmenopausal women with osteoporosis did not reveal any differences between those groups at increased risk and the high-risk population [42]. However, most analyses have suggested improved results in high-risk subgroups, including clinical studies of teriparatide [43] and denosumab [44]. In contrast, a few studies have suggested the reverse effect, including the randomised, blinded, placebo-controlled MORE study, which demonstrated that raloxifene had a greater effect in reducing vertebral fracture risk in postmenopausal women with low BMD and no pre-existing fractures versus those with pre-existing fractures at baseline [45]. Differences were also observed when subgroups for analyses were defined using different criteria. For example, in the randomised, placebo-controlled HIP study, risedronate reduced the risk of hip fracture in the subgroup of women with confirmed osteoporosis, but not among women selected primarily based on non-skeletal risk factors other than low BMD [46].
There are, however, some inherent limitations to subgroup analyses. These include the loss of statistical power compared with the original study design, which can lead to a high risk of false-positive results and misinterpretation, and the fact that the results are also dependent on the cut-off point used for analysis. In most analyses, even when results appear different between subgroups, interaction tests were non-significant. It should therefore be highlighted that primary endpoint results provide the greatest weight of evidence in clinical studies, whereas subgroup analyses, particularly when undertaken posthoc, can provide useful information, but are lower in the hierarchy of evidence grades.
Some of the pitfalls of subgroup analyses can be avoided by assessing the efficacy of a treatment as a continuous function of fracture risk using BMD or FRAX [47]. To date, results from posthoc analyses exploring the relationship between the efficacy of osteoporosis medication and baseline fracture probabilities assessed with FRAX have shown differences between interventions. Studies of treatment with clodronate [48], bazedoxifene [49] and denosumab [50] have shown larger reductions in fracture incidence with increasing baseline FRAX fracture probability in postmenopausal women. The finding of greater efficacy at higher fracture probabilities has important implications for health technology assessments, as treatments should ideally be targeted to high-risk patients so greater efficacy in the higher-risk groups could improve the budget impact and the cost-effectiveness of interventions. In contrast, other studies assessing treatment with raloxifene [49, 51], strontium ranelate [52], abaloparatide [53] and teriparatide [54, 55] have shown no significant interaction between treatment efficacy and baseline fracture probability assessed using FRAX.
In terms of future research, further clinical efficacy data supported by health economic assessments are needed so that clinicians, regulatory bodies and payers can identify which treatments are most effective in terms of clinical efficacy, safety and cost for those patients at increased risk of fracture [56, 57]. To date, few studies have evaluated the effect of treatment in patients with an increased risk of fracture immediately following a first event of fracture, a period during which they are at highest risk. Importantly, the HORIZON study demonstrated that infusion of zoledronic acid ≥2 weeks after hip fracture repair resulted in hip BMD increases, significant reductions in subsequent vertebral, non-vertebral and hip fracture risk and reduced mortality [58]. Further studies are also needed to assess the cost-effectiveness of early secondary prevention strategies in addition to the clinical outcomes. Similarly, few studies have yet demonstrated the efficacy of treatment in patients with an increased risk of fracture related to a high risk of falls.
Given the current lack of clear evidence and the difficulties in identifying treatments that have the best outcomes in patients at increased fracture risk, it is recommended that current treatments for osteoporosis should demonstrate efficacy in reducing the risk of both vertebral and non-vertebral fractures.
What can we expect from emerging agents for the management of patients at increased risk of fracture?
Although there is a range of osteoporosis treatments available, as previously noted, some have only moderate efficacy, are associated with significant side effects and/or require regular and somewhat complex dosing regimens [27, 59]. Thus, there is still an unmet need for new agents that provide more effective fracture prevention and are well tolerated, particularly in those patients who have had a prior fracture and are at increased risk of a subsequent fracture during the first year. As noted in a recent review of future approaches for patients at high risk of hip fracture, any new pharmacotherapy should ideally aim to restore both trabecular and cortical bone strength and be used in addition to lifestyle interventions, fall prevention and potentially surgical intervention [59–61].
One drug currently under regulatory review is abaloparatide, a synthetic analogue of human PTH-related protein (PTHrP), which acts as a selective activator of the PTH type 1 receptor (PTHR1) signalling pathway [62]. Abaloparatide has been investigated in the randomised, double-blind, placebo-controlled, phase 3 ACTIVE study, which included an open-label teriparatide comparator arm [39]. In a population of postmenopausal osteoporotic women, abaloparatide given for 18 months significantly reduced the risk of new vertebral fractures (primary endpoint) by 86% compared with placebo (Fig. 6a), and significantly reduced the risk of non-vertebral fractures, MOFs and clinical fractures by 43, 70 and 43%, respectively. Abaloparatide was also associated with modestly higher BMD gains, particularly at cortical bone-rich sites, compared with teriparatide. The incidence of hypercalcaemia was lower with abaloparatide than teriparatide, consistent with the postulated lower bone resorption with abaloparatide, and no differences in adverse events were observed between the treatment groups.
a Number of fractures at the sites shown in patients given placebo (n = 825), teriparatide (n = 818) or abaloparatide (n = 824) after 18 months of treatment [39] (abaloparatide significantly different from placebo; romosozumab significantly different from placebo; risk ratio 0.27; P < 0.001.). b Incidence of new vertebral fracture following treatment with romosozumab [63] (abaloparatide significantly different from teriparatide; romosozumab significantly different from teriparatide; risk ratio 0.25; P < 0.001). The risk ratio was assessed among patients in the romosozumab group compared with those in the placebo group at 12 months (end of the double-blind period) and at 24 months (by which time patients in both groups had received open-label denosumab for 12 months)
Romosozumab is another osteo-anabolic compound under investigation for the management of osteoporosis. It is a monoclonal antibody that binds to and inhibits sclerostin, a glycoprotein produced by osteocytes that has an important role as a regulator of bone formation due to its inhibitory actions on the Wnt signalling pathway [64]. The phase 3, randomised, double-blind, placebo-controlled FRAME study evaluated treatment with romosozumab in postmenopausal osteoporotic women [63]. Patients were randomised to romosozumab or placebo for 12 months; thereafter, all patients received open-label denosumab for a further 12 months. Monthly subcutaneous injections of romosozumab were found to reduce the incidence of new vertebral fractures at 12 and 24 months compared with placebo. At 12 months, romosozumab significantly reduced the risk of new vertebral fractures and clinical fractures by 73 and 36%, respectively, compared with placebo (Fig. 6b). Romosozumab also reduced the risk of non-vertebral fractures by 25%, although this was not significantly different versus placebo. When patients from low-risk countries were excluded, significant effects on non-vertebral fractures were observed, suggesting that there may be a significant interaction between fracture probability and efficacy. The cumulative 24-month incidence of new vertebral fracture was significantly lower in the group previously treated with romosozumab than in those treated with placebo, although no significant difference in non-vertebral fracture was observed. Romosozumab was also associated with increases in BMD at the lumbar spine, total hip and femoral neck compared with placebo at 12 months, and these gains were further increased after transition to denosumab. Adverse events were balanced across the treatment groups, and although serious hypersensitivity reactions were observed with romosozumab, these were uncommon. Rare cases of osteonecrosis of the jaw and atypical femoral fracture were observed, although they had confounding factors that may have contributed to the events. The development of romosozumab therefore constitutes another promising option for the management of osteoporosis in high-risk patients.
Conclusions
Osteoporosis represents a significant healthcare burden in European countries which, due to the increasing number of elderly people, is predicted to rise further in the future. Despite this outlook, most patients at increased risk of fracture, such as those with a prior fracture, do not receive medication, resulting in a large treatment gap that urgently needs to be addressed. Identification of other potential fracture risk factors and of patients who are at particularly high risk of fracture during a certain time period, e.g. in the year after a fracture, will help clinicians target appropriate treatment more precisely and cost-effectively, and should be the focus of future research and clinical study endpoints. The new therapeutic bone-forming agents that are currently in development and have shown promising results will hopefully add to the treatment options available to clinicians.
Early adoption of effective fracture prevention strategies targeted to patients at increased risk of fracture is critical to reducing the healthcare burden of osteoporosis. Ongoing global initiatives, such as the International Osteoporosis Foundation’s ‘Capture the Fracture®’ campaign, aim to develop a best practice framework that will act as an international benchmark for fracture liaison services throughout the world, and endeavour to provide support and resources that will facilitate their implementation at a local and national level. Ultimately, such strategies will help close the current gap in secondary fracture prevention.
Change history
07 August 2017
An erratum to this article has been published.
References
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Beaudart C, McCloskey E, Bruyere O et al (2016) Sarcopenia in daily practice: assessment and management. BMC Geriatr 16:170
Freemantle N, Cooper C, Roux C et al (2010) Baseline observations from the POSSIBLE EU® study: characteristics of postmenopausal women receiving bone loss medications. Arch Osteoporos 5:61–72
Johansson H, Odén A, McCloskey EV, Kanis JA (2014) Mild morphometric vertebral fractures predict vertebral fractures but not non-vertebral fractures. Osteoporosis Int 25:235–241
Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporosis Int 23:2239–2256
Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA, Epidemiology ICWGoF (2011) Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 22:1277–1288
Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, de Vries F, van Staa TP, Cooper C, Harvey NC (2016) Epidemiology of fractures in the United Kingdom 1988-2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone 87:19–26
Johnell O, Borgstrom F, Jonsson B, Kanis J (2007) Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int 18:333–337
Lucas RM, Severo A, Silva M, Monjardino P, Gaio T, Cooper AR, Barros H (2017) Is there a shared hip fracture epidemic in Europe? Modelling recent time trends in 14 countries. Submitted for publication
Rosengren BE, Bjork J, Cooper C, Abrahamsen B (2016) Recent hip fracture trends in Sweden and Denmark with age-period-cohort effects. Osteoporos Int 28:139–149
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Kanis JA, Borgstrom F, Compston J, Dreinhofer K, Nolte E, Jonsson L, Lems WF, McCloskey EV, Rizzoli R, Stenmark J (2013) SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 8:144
Ioannidis G, Flahive J, Pickard L et al (2013) Non-hip, non-spine fractures drive healthcare utilization following a fracture: the Global Longitudinal Study of Osteoporosis in Women (GLOW). Osteoporos Int 24:59–67
Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175–179
Johnell O, Oden A, Caulin F, Kanis JA (2001) Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int 12:207–214
Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P (2009) Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J Bone Miner Res 24:1299–1307
Greenspan SL, Wyman A, Hooven FH et al (2012) Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc 60:455–461
Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD (2014) Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res 29:1929–1937
Carnevale V, Nieddu L, Romagnoli E, Bona E, Piemonte S, Scillitani A, Minisola S (2006) Osteoporosis intervention in ambulatory patients with previous hip fracture: a multicentric, nationwide Italian survey. Osteoporos Int 17:478–483
Fraser LA, Ioannidis G, Adachi JD et al (2011) Fragility fractures and the osteoporosis care gap in women: the Canadian Multicentre Osteoporosis Study. Osteoporos Int 22:789–796
Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster JY, Rizzoli R, Cooper C, Kanis JA (2017) Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int
Khosla S, Cauley JA, Compston J, Kiel DP, Rosen C, Saag KG, Shane E (2016) Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res 32:424–43
van der Velde RY, Wyers CE, Teesselink E, Geusens PP, van den Bergh JP, de Vries F, Cooper C, Harvey NC, van Staa TP (2017) Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: variation by age, sex, geographic location and ethnicity. Bone 94:50–55
Wysowski DK, Greene P (2013) Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002-2012. Bone 57:423–428
Landfeldt E, Strom O, Robbins S, Borgstrom F (2012) Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 23:433–443
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, McCloskey EV (2016) A systematic review of intervention thresholds based on FRAX. Arch Osteoporos 11:25
Cummings SR, Cosman F, Lewiecki EM et al (2016) Goal-directed treatment for osteoporosis: a progress report from the ASBMR-NOF Working Group on Goal-Directed Treatment for Osteoporosis. J Bone Miner Res 32:3–10
Kanis JA, Svedbom A, Harvey N, McCloskey EV (2014) The osteoporosis treatment gap. J Bone Miner Res 29:1926–1928
McCloskey E, Lenaghan E, Clark S et al (2016) Screening based on FRAX fracture risk reduces the incidence of hip fractures in older community-dwelling women—results from the SCOOP study. Osteoporos Int 27:S624
Nakayama A, Major G, Holliday E, Attia J, Bogduk N (2016) Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int 27:873–879
Leal J, Gray AM, Hawley S, Prieto-Alhambra D, Delmestri A, Arden NK, Cooper C, Javaid MK, Judge A, group REs (2016) Cost-effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population based study. J Bone Miner Res Sep 16
Javaid MK, Kyer C, Mitchell PJ et al (2015) Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture® Best Practice Framework tool. Osteoporos Int 26:2573–2578
Rizzoli R, Branco J, Brandi ML et al (2014) Management of osteoporosis of the oldest old. Osteoporos Int 25:2507–2529
Lloyd BD, Williamson DA, Singh NA et al (2009) Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci 64:599–609
Giangregorio LM, Leslie WD, Manitoba Bone Density P (2010) Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res 25:1400–1405
Kanis JA, Rizzoli R, Cooper C, Reginster JY (2014) Challenges for the development of bone-forming agents in Europe. Calcif Tissue Int 94:469–473
Miller PD, Hattersley G, Riis BJ et al (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316:722–733
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Reginster JY, Seeman E, Vernejoul MCD et al (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90:2816–2822
Cosman F, Hattersley G, Hu MY, Williams GC, Fitzpatrick LA, Black DM (2017) Effects of abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res 32:17–23
Gallagher JC, Genant HK, Crans GG, Vargas SJ, Krege JH (2005) Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab 90:1583–1587
Boonen S, Adachi JD, Man Z et al (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 96:1727–1736
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33:522–532
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women: Hip Intervention Program Study Group. N Engl J Med 344:333–340
Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G (2001) Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 5:1–56
McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, Jalava T, Kanis JA (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy—additional results from a double-blind, placebo-controlled randomised study. Osteoporosis Int 20:811–817
Kanis JA, Johansson H, Oden A, McCloskey EV (2009) Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 44:1049–1054
McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, Lewiecki EM, Lorenc R, Libanati C, Kanis JA (2012) Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 27:1480–1486
Kanis JA, Johansson H, Oden A, McCloskey EV (2010) A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone 47:729–735
Kanis JA, Johansson H, Oden A, McCloskey EV (2011) A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX. Osteoporos Int 22:2347–2355
McCloskey EV, Johansson H, Harvey NC, Oden A, Jiang H, Modin S, Fitzpatrick L, Kanis JA (2016) Effect of investigational treatment abaloparatide-SC for prevention of major osteoporotic fracture or any fracture is independent of baseline fracture probability. J Bone Miner Metab 31:S381
Harvey NC, Kanis JA, Oden A, Burge RT, Mitlak BH, Johansson H, McCloskey EV (2015) FRAX and the effect of teriparatide on vertebral and non-vertebral fracture. Osteoporos Int 26:2677–2684
Harvey NC, Kanis JA, Odén A, Nakamura T, Shiraki M, Sugimoto T, Kuroda T, Johansson H, McCloskey EV (2015) Efficacy of weekly teriparatide does not vary by baseline fracture probability calculated using FRAX. Osteoporosis Int 26:2347–2353
Hiligsmann M, Evers SM, Ben Sedrine W, Kanis JA, Ramaekers B, Reginster JY, Silverman S, Wyers CE, Boonen A (2015) A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. PharmacoEconomics 33:205–224
Hiligsmann M, Kanis JA, Compston J et al (2013) Health technology assessment in osteoporosis. Calcif Tissue Int 93:1–14
Eriksen EF, Lyles KW, Colon-Emeric CS et al (2009) Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res 24:1308–1313
Ferrari S, Reginster JY, Brandi ML, Kanis JA, Devogelaer JP, Kaufman JM, Feron JM, Kurth A, Rizzoli R (2016) Unmet needs and current and future approaches for osteoporotic patients at high risk of hip fracture. Arch Osteoporos 11:37
Harvey NC, Biver E, Kaufman JM et al (2017) The role of calcium supplementation in healthy musculoskeletal ageing : an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 28:447–462
Rizzoli R, Stevenson JC, Bauer JM et al (2014) The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 79:122–132
Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ (2016) Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 157:141–149
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Ominsky MS, Boyce RW, Li X, Ke HZ (2016) Effects of sclerostin antibodies in animal models of osteoporosis. Bone Oct 24
Acknowledgements
The working group and this paper were fully funded by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), a Belgian not-for-profit organisation. ESCEO was responsible for the selection of participants in the preliminary meeting and for the choice of the authors of the manuscript, covering all expenses related to the organisation of the meeting. ESCEO also covered all expenses pertaining to the preparation, writing and submission of the manuscript. ESCEO received unrestricted educational grants from different non-governmental organisations, not-for-profit organisations and commercial partners. None of the partners were involved in the organisation of the ESCEO working group, which prepared this manuscript, and were not part of the writing or review team of the manuscript. Editorial assistance for the development of this manuscript was provided by Adelphi Communications Ltd., UK, and funded by ESCEO.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
JAK reports grants from Amgen, Eli Lilly and Radius Health; non-financial support from Medimaps, and Asahi; and other support from AgNovos outside the submitted work. JAK is the architect of FRAX but has no financial interest. CC has received consultancy, lecture fees and honoraria from Amgen, GlaxoSmithKline, Alliance for Better Bone Health, Merck Sharp & Dohme, Eli Lilly, Pfizer, Novartis, Servier, Medtronic and Roche. RR has received consulting fees or advisory board fees from Radius Health, Labatec, Danone and Nestlé. BA has institutional research contracts with Novartis and UCB outside of the submitted work. NMA-D has received support from the Prince Mutaib Chair for Biomarkers of Osteoporosis, Deanship of Scientific Research Chairs, King Saud University. MLB has received consultancy fees and grants from Alexion, Abiogen, Amgen, Eli Lilly and Shire. JC-A has received grants and/or advisory board fees from Amgen, Servier, Fresenius-VIFOR and Shire. BC has received consultancy fees, lecture fees and honorarium from Amgen, Eli Lilly, Expanscience, Ferring, Medtronic, Novartis, Roche Diagnostics and Servier. HPD has received lecture fees, consulting fees and/or advisory board fees from Amgen, Daiichi-Sankyo, Eli Lilly, Genericon, Kyphon, Merck Sharp & Dohme, Novartis, Nycomed, Servier and Sinapharm. SF has received grants or research support from Amgen and Merck Sharp & Dohme and consultancy fees from Amgen, Merck Sharp & Dohme, GlaxoSmithKline, Eli Lilly and UCB. PH has received grants, advisory board or speaker fees from Amgen, AstraZeneca, Eli Lilly, Exeltis, Daichii-Sankyo, Gedeon Richter, Meda, Merck Sharp & Dohme, Mylan, Novartis, Pfizer, Roche and UCB. NCH has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, Amgen, Merck Sharp & Dohme, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare and Internis Pharma. MK has no conflicts of interest to declare. AK has received consulting and speaker fees from Agnovos, Amgen, Eli Lilly, Novartis, Novo Nordisk, Roche, Servier, Biomet and Dfine, Inc. EM is or has acted as a consultant, advisor, speaker and/or received research support from ActiveSignal, Amgen, Arthritis Research UK, AstraZeneca, Consilient Healthcare, EPSRC, GlaxoSmithKline, Hologic, I3 Innovus, Internis, the International Osteoporosis Foundation, Eli Lilly, the Medical Research Council, Medtronic, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, Synexus, Tethys, UCB, Unilever and Warner Chilcott. SM has served as a speaker for Abiogen, Amgen, Diasorin, Eli Lilly, Italfarmaco, Fujii, Merck Sharp & Dohme and Takeda and on advisory boards for Amgen and Eli Lilly. TT has received advisory board or speaker fees from Amgen, Chugai/Roche, Expanscience, Genévrier, GlaxoSmithKline, HAC-Pharma, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Teva and UCB and research grants or investigator fees from Amgen, Bone Therapeutics, Chugai/Roche, LCA, Merck Sharp & Dohme, Novartis, Pfizer and UCB. J-YR has received advisory board or speaker fees from Asahi-Kasei, Eli Lilly, IBSA-Genévrier, Nycomed-Takeda, PharmEvo, Radius Health, Roche, Servier, UCB, Will Pharma and Zodiac.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kanis, J.A., Cooper, C., Rizzoli, R. et al. Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 28, 2023–2034 (2017). https://doi.org/10.1007/s00198-017-4009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4009-0