Abstract
Summary
The majority of tumor-induced osteomalacia cases have been reported in the Northern Hemisphere and Asia. In this first series of South American patients, we show that the clinical presentation and sensitivity of plasmatic fibroblast growth factor 23 and somatostatin analog-based imaging are similar to those described in other populations.
Introduction
Describe the experience of clinical presentation, diagnostic study, and treatment of patients with tumor-induced osteomalacia (TIO) in a South American academic center in comparison to literature.
Methods
Analysis of the records of patients diagnosed with TIO. The clinical presentation, diagnostic studies, and treatment were analyzed. Fibroblast growth factor 23 (FGF23) was measured by ELISA.
Results
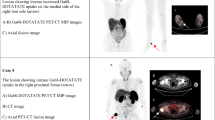
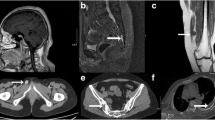
Six patients were diagnosed with TIO during the studied period. The patients’ median age was 53 years (range 22–64). All patients presented with weakness and pain in the extremities. Four experienced fractures during their evolution. The median time to diagnosis was 4.5 years (1–20). Biochemical studies showed hypophosphatemia, median of 1.4 mg/dL (1.2–1.6), with low maximum rates of tubular reabsorption of phosphate adjusted for glomerular filtration rate. FGF23 was elevated in 4/6 patients and inappropriately normal in the other two. In three patients, the location of the tumor was clinically evident and confirmed with anatomical imaging. In the remaining patients, two tumors were located with 68Ga DOTATATE-PET/CT and one with OctreoScan. The causal tumors were located in the lower extremities in five patients and invading the frontal sinus in one patient. In all patients, tumors were successfully removed. Within 14 days, there was normalization of phosphate and FGF23 levels and resolution of clinical symptoms in all patients. In all cases, the histopathology was compatible with a phosphaturic mesenchymal tumor.
Conclusions
The clinical presentation, delay time to diagnosis, FGF23 diagnostic sensitivity and histopathology in this first series of South American patients is similar to those described in other populations. The success of localization by somatostatin analog-based imaging, suggests this may the optimal imaging modality.




Similar content being viewed by others
References
Chong WH, Molinolo AA, Chen CC, Collins MT (2011) Tumor-induced osteomalacia. Endocr Relat Cancer 18:R53–R77
Jan de Beur SM (2005) Tumor-induced osteomalacia. JAMA 294:1260–1267
Tsagalis G, Psimenou E, Manios E, Laggouranis A (2009) Fibroblast growth factor 23 (FGF23) and the kidney. Int J Artif Organs 32:232–239
Bhattacharyya N, Chong WH, Gafni RI, Collins MT (2012) Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab 23:610–618
Fukumoto S (2008) Physiological regulation and disorders of phosphate metabolism—pivotal role of fibroblast growth factor 23. Intern Med 47:337–343
Jonsson KB, Zahradnik R, Larsson T et al (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348:1656–1663
Jiang Y, Xia WB, Xing XP et al (2012) Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res 27:1967–1975
Haeusler G, Freilinger M, Dominkus M, Egerbacher M, Amann G, Kolb A, Schlegel W, Raimann A, Staudenherz A (2010) Tumor-induced hypophosphatemic rickets in an adolescent boy—clinical presentation, diagnosis, and histological findings in growth plate and muscle tissue. J Clin Endocrinol Metab 95:4511–4517
Mussig K, Oksuz MO, Pfannenberg C, Adam P, Zustin J, Beckert S, Petersenn S (2009) Somatostatin receptor expression in an epitheloid hemangioma causing oncogenic osteomalacia. J Clin Endocrinol Metab 94:4123–4124
Clifton-Bligh RJ, Hofman MS, Duncan E et al (2013) Improving diagnosis of tumor-induced osteomalacia with gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab 98:687–694
El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R, Millo C, Chen CC, Kebebew E, Collins MT (2016) 68Ga-DOTATATE for tumor localization in tumor-induced Osteomalacia. J Clin Endocrinol Metab 101:3575–3581
Zhang J, Zhu Z, Zhong D et al (2015) 68Ga DOTATATE PET/CT is an accurate imaging modality in the detection of culprit tumors causing Osteomalacia. Clin Nucl Med 40:642–646
Bhavani N, Reena Asirvatham A, Kallur K, Menon AS, Pavithran PV, Nair V, Vasukutty JR, Menon U, Kumar H (2016) Utility of gallium-68 DOTANOC PET/CT in the localization of tumour-induced osteomalacia. Clin Endocrinol 84:134–140
Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, Bhansali A (2015) Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med 40:e6–e10
Jadhav S, Kasaliwal R, Lele V et al (2014) Functional imaging in primary tumour-induced osteomalacia: relative performance of FDG PET/CT vs somatostatin receptor-based functional scans: a series of nine patients. Clin Endocrinol 81:31–37
Serafini EM, Pisarevsky AA, Plumet Garrido J, Zamora RJ, Petrucci EA (2013) Tumor-induced osteomalacia: rhinosinusal hemangiopericytoma. Medicina (B Aires) 73:39–42
Sanchez A, Castiglioni A, Coccaro N, Silva R, Bobrovsky E, Moyses RMA, Graciolli F (2013) Ostemalacia due to a tumor secreting FGF-23. Medicina-Buenos Aire 73:43–46
Jerkovich F, Moncet D, Babini S, Zoppi JA, Graciolli F, Oliveri B (2015) Oncogenic osteomalacia. Report of two cases. Medicina-Buenos Aire 75:37–40
Romualdo-Silva DD, Silva BC, Caetano CV, Tiburcio AM, Nunes MB, Chagas SA, Polito ET, Ferreira AR, Purisch S (2009) Tumor-induced osteomalacia: a case report. Arq Bras Endocrinol Metabol 53:378–382
Biagini GL, Coutinho PR, Jonasson TH, Ueda CE, Gama RR (2008) Oncogenic osteomalacia: localization of underlying peripheral tumor with 99mTc-sestamibi scintigraphy. Arq Bras Endocrinol Metabol 52:1505–1509
Andrade TSF, Gonzalez C, Cabezon R (2013) Tumor Mesenquimatico fosfaturico de fosa nasal con compromiso intracraneano: Reporte de un caso y revision de la literatura. Rev Otorrinolaringol Cir Cabeza Cuello 73:57–62
von Falck C, Rodt T, Rosenthal H, Langer F, Goesling T, Knapp WH, Galanski M (2008) (68)Ga-DOTANOC PET/CT for the detection of a mesenchymal tumor causing oncogenic osteomalacia. Eur J Nucl Med Mol Imaging 35:1034
Roarke MC, Nguyen BD (2007) PET/CT localization of phosphaturic mesenchymal neoplasm causing tumor-induced osteomalacia. Clin Nucl Med 32:300–301
Hesse E, Moessinger E, Rosenthal H, Laenger F, Brabant G, Petrich T, Gratz KF, Bastian L (2007) Oncogenic osteomalacia: exact tumor localization by co-registration of positron emission and computed tomography. J Bone Miner Res 22:158–162
Khadgawat R, Singh Y, Kansara S, Tandon N, Bal C, Seith A, Kotwal P (2009) PET/CT localisation of a scapular haemangiopericytoma with tumour-induced osteomalacia. Singap Med J 50:e55–e57
Chua SC, O’Connor SR, Wong WL, Ganatra RH (2008) Case report: solitary plasmacytoma of bone with oncogenic osteomalacia: recurrence of tumour confirmed by PET/CT. A case report with a review of the radiological literature. Br J Radiol 81:e110–e114
Jagtap VS, Sarathi V, Lila AR, Malhotra G, Sankhe SS, Bandgar T, Menon P, Shah NS (2011) Tumor-induced osteomalacia: a single center experience. Endocr Pract 17:177–184
Dupond JL, Magy N, Mahammedi M, Prie D, Gil H, Meaux-Ruault N, Kantelip B (2005) Oncogenic osteomalacia: the role of the phosphatonins. Diagnostic usefulness of the fibroblast growth factor 23 measurement in one patient. Rev Med Interne 26:238–241
Imel EA, Peacock M, Pitukcheewanont P et al (2006) Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 91:2055–2061
Endo I, Fukumoto S, Ozono K et al (2008) Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone 42:1235–1239
Halperin F, Anderson RJ, Mulder JE (2007) Tumor-induced osteomalacia: the importance of measuring serum phosphorus levels. Nat Clin Pract Endocrinol Metab 3:721–725
Imel EA, Econs MJ (2012) Approach to the hypophosphatemic patient. J Clin Endocrinol Metab 97:696–706
Farrow EG, White KE (2009) Tumor-induced osteomalacia. Expert Rev Endocrinol Metab 4:435–442
Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT (2007) Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 22:931–937
Aono Y, Hasegawa H, Yamazaki Y, Shimada T, Fujita T, Yamashita T, Fukumoto S (2011) Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J Bone Miner Res 26:803–810
Imel EA, Zhang X, Ruppe MD et al (2015) Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab 100:2565–2573
Kinoshita Y, Fukumoto S (2014) Anti-FGF23 antibody therapy for patients with tumor-induced osteomalacia. Clin Calcium 24:1217–1222
Acknowledgments
We thank the support of the Research Direction of the Faculty of Medicine, Pontificia Universidad Catolica de Chile in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
González, G., Baudrand, R., Sepúlveda, M.F. et al. Tumor-induced osteomalacia: experience from a South American academic center. Osteoporos Int 28, 2187–2193 (2017). https://doi.org/10.1007/s00198-017-4007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4007-2