Abstract
Summary
Alendronate therapy has been associated with serious side effects. Altering the alendronate concentration and combining with high-frequency loading as mechanical intervention was explored in this animal study as a treatment for osteoporosis. The bone anabolic potency of high-frequency loading was overruled by the different alendronate dosages applied in the present study. Further exploration of reduced hormonal therapy associated with mechanical interventions in osteoporosis treatment should be sought.
Introduction
The aim of the present study was to investigate the effect of alendronate (ALN) administration at two different dosages, associated or not with high-frequency (HF) loading, on the bone microstructural response.
Methods
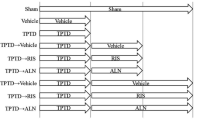
Sixty-four female Wistar rats were used, of which 48 were ovariectomized (OVX) and 16 were sham-operated (shOVX). The OVX animals were divided into three groups: two groups were treated with alendronate, at a dosage of 2 mg/kg (ALN(2)) or at a reduced dosage of 1 mg/kg (ALN(1)) three times per week. A third OVX group did not receive pharmaceutical treatment. All four groups were mechanically stimulated via whole body vibration (WBV) at HF (up to 150 Hz) or left untreated (shWBV). ALN and HF were administered for 6 weeks, starting at 10-week post-(sh)OVX. Tibia bone structural parameters were analyzed using ex vivo microcomputed tomography.
Results
Trabecular bone loss and structural deterioration resulting from ovariectomy were partially restored by ALN administration, demonstrated by the improvement of trabecular patter factor (Tb.Pf), trabecular separation (Tb.Sp), and structure model index (SMI) of the ALN groups compared to that of the OVX group, regardless of the applied dosage [ALN(2) or ALN(1)] or mechanical loading regime (shWBV or WBV). However, a significant positive effect of the ALN(1) administration on trabecular (decrease of Tb.Sp and SMI) and cortical bone (increase of cortical thickness) microarchitecture compared to that of the OVX status group was observed for both loading regimes was not seen for ALN(2). Furthermore, HF loading resulted in cortical bone changes, with an increased trabeculary area and endocortical perimeter. Finally, the benefits of a combined therapy of ALN with HF loading could not be discerned in the present experimental conditions.
Conclusions
The bone anabolic potency of HF loading was overruled by the ALN dosages applied in the present study. Further altering the ALN dosage combined with robust mechanical stimuli needs to be considered in osteoporosis research and eventually therapy.




Similar content being viewed by others
References
Rachner T, Khosla S, Hofbauer L, Manuscript A (2011) New horizons in osteoporosis. Lancet 377:1276–1287
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:1–115
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 322:305–311
Iwamoto J, Sato Y, Takeda T, Matsumoto H (2012) Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT). J Musculoskelet Neuronal Interact 12:136–143
Roschger P, Misof B, Paschalis E, Fratzl P, Klaushofer K (2014) Changes in the degree of mineralization with osteoporosis and its treatment. Curr Osteoporos Rep 12:338–350
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American Association of Oral and Maxillofacial Surgeons Position Paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. J Oral Maxillofac Surg 67:2–12
Im S, Lim SH, Lee JI et al (2010) Effective dosage and administration schedule of oral alendronate for non-nociceptive symptoms in rats with chronic constriction injury. J Korean Med Sci 25:938–944
Chang CH, Wang CZ, Chang JK, Hsu CY, Ho ML (2014) The susceptive alendronate-treatment timing and dosage for osteogenesis enhancement in human bone marrow-derived stem cells. PLoS One 9:1–9
Chatterjee M, Hatori K, Duyck J, Sasaki K, Naert I, Vandamme K (2014) High-frequency loading positively impacts titanium implant osseointegration in impaired bone. Osteoporos Internat 26:281–290
Hatori K, Camargos GV, Chatterjee M et al (2015) Single and combined effect of high-frequency loading and bisphosphonate treatment on the bone micro-architecture of ovariectomized rats. Osteoporos Int 26:303–313
Chen B, Li Y, Yang X, Xie D (2013) Comparable effects of alendronate and strontium ranelate on femur in ovariectomized rats. Calcif Tissue Internat 93:481–486
Judex S, Lei X, Han D, Rubin C (2007) Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40:1333–1339
Giro G, Gonçalves D, Sakakura CE, Pereira RMR, Marcantonio Júnior E, Orrico SRP (2008) Influence of estrogen deficiency and its treatment with alendronate and estrogen on bone density around osseointegrated implants: radiographic study in female rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:162–167
Liu XL, Li CL, Lu WW, Cai WX, Zheng LW (2015) Skeletal site-specific response to ovariectomy in a rat model: change in bone density and microarchitecture. Clin Oral Implants Res 26:392–398
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23:576–581
Washimi Y, Ito M, Morishima Y et al (2007) Effect of combined humanPTH(1-34) and calcitonin treatment in ovariectomized rats. Bone 41:786–793
Yao W, Cheng Z, Koester KJ et al (2007) The degree of bone mineralization is maintained with single intravenous bisphosphonates in aged estrogen-deficient rats and is a strong predictor of bone strength. Bone 41:804–812
Campbell GM, Bernhardt R, Scharnweber D, Boyd SK (2011) The bone architecture is enhanced with combined PTH and alendronate treatment compared to monotherapy while maintaining the state of surface mineralization in the OVX rat. Bone 49:225–232
Brouwers JEM, Lambers FM, Gasser JA, van Rietbergen B, Huiskes R (2008) Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized wistar rats assessed by in vivo micro-computed tomography. Calcif Tissue Int 82:202–211
Boyd SK, Davison P, Muller R, Gasser JA (2006) Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 39:854–862
Yang J, Pham SM, Crabbe DL (2003) Effects of oestrogen deficiency on rat mandibular and tibial microarchitecture. Dentomaxillofac Radiol 32:247–251
Waarsing JH, Day JS, Verhaar JAN, Ederveen AGH, Weinans H (2006) Bone loss dynamics result in trabecular alignment in aging and ovariectomized rats. J Orthop Res 24:926–935
Frost HM (2003). Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol.Cell Evol Biol 275:1081–1101
Seeman E (2013) Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci 68:1218–1225
Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L (2003) Endocrinology: bone adaptation requires oestrogen receptor-α. Nature 424:389–389
Lee KCL, Lanyon LE (2004) Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev 32:64–68
Lim SK, Won YJ, Lee HC, Huh KB, Park YS (1999) A PCR analysis of ERalpha and ERbeta mRNA abundance in rats and the effect of ovariectomy. J Bone Min Res 14:1189–1196
Saxon LK, Turner CH (2005) Estrogen receptor beta: the antimechanostat? Bone 36:185–192
Rubinacci A, Marenzana M, Cavani F et al (2008) Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int 82:316–326
You L, Sheng Z, Chen J (2011) The safety and efficacy of early-stage bi-weekly alendronate to improve bone mineral density and bone turnover in Chinese post-menopausal women at risk of osteoporosis. J Int Med Res 39:302–310
Acknowledgments
The authors would like to acknowledge Dr. A. Ivanova for the help with the statistical analysis. This work was supported by the Fund for Scientific Research Flanders (FWO-Vlaanderen) by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (2014/08912-1 for Postdoctoral researcher Correa CB) and the Brazilian Science Without Borders Program (246131/2012-8 for PhD student Camargos GV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of the animal experiment was approved by the ethical committee of KU Leuven (P050/2011), complied with ARRIVE guidelines for preclinical studies and was performed according to the Belgian animal welfare regulations and guidelines.
Conflicts of interest
The authors Cassia Bellotto Correa, Germana De Villa Camargos, Marissa Chatterjee, Marcelo Ferraz Mesquita, Altair Antoninha Del Bel Cury, Ignace Naert, Joke Duyck, and Katleen Vandamme declare that there are no conflicts of interest related to the manuscript.
Additional information
Correa CB and Camargos GV shared first authorship.
Rights and permissions
About this article
Cite this article
Correa, C., Camargos, G., Chatterjee, M. et al. Can the alendronate dosage be altered when combined with high-frequency loading in osteoporosis treatment?. Osteoporos Int 28, 1287–1293 (2017). https://doi.org/10.1007/s00198-016-3859-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3859-1