Abstract
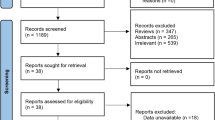
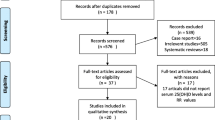
Our meta-analysis assessed the efficacy of statins on the risk of fracture, bone mineral density (BMD), and the markers of bone metabolism by collecting data from 33 clinical trials. We found that statin treatment was associated with bone metabolism. And statins seemed to be more effective on male patients with osteoporosis. The efficacy of statins for the treatment of osteoporosis has been controversial in previous studies and meta-analyses. Our meta-analysis was conducted to examine in detail the efficacy of statins on osteoporosis. We searched PubMed, Embase, and the Cochrane Library databases for clinical trials from inception to May 2016. We included studies that described the effect of statins on the risk of fracture, BMD, or bone turnover markers. Moreover, we also conducted subgroup analyses according to the skeleton site, patient gender, and length of follow-up. A total of 33 studies which included 23 observational studies (16 cohort studies and 7 case-control studies) and 10 randomized controlled trials (RCTs) were evaluated. These 33 studies included 314,473 patients in statin group and 1,349,192 patients in control group. Statins decreased the risk of overall fractures (OR = 0.81, 95% CI 0.73–0.89) and hip fractures (OR = 0.75, 95% CI 0.60–0.92). Furthermore, the use of statins was associated with increased BMD at the total hip (standardized mean difference (SMD) = 0.18, 95% CI 0.00–0.36) and lumbar spine (SMD = 0.20, 95% CI 0.07–0.32) and improved the bone formation marker, osteocalcin (OC) (SMD = 0.21, 95% CI 0.00–0.42). However, there was no positive effect on vertebral fractures, upper extremity fractures, BMD at the femoral neck, bone-specific alkaline phosphatase (BALP), and serum C-terminal peptide of type I collagen (S-CTX). Also, compared with male subgroups, the effect on female subgroups was only slightly positive or of no statistical significance. Our meta-analysis indicates that statin treatment may be associated with a decreased risk of overall fractures and hip fractures, an increased BMD at the total hip, BMD at the lumbar spine, and OC. Moreover, our results also show that statin treatment may have a greater effect on male patients than on female patients.




Similar content being viewed by others
References
Famili P, Cauley J, Suzuki JB, Weyant R (2005) Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 76(1):11–15
O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ (1996) The prevalence of vertebral deformity in European men and women: the European vertebral osteoporosis study. J Bone Miner Res 11(7):1010–1018
Dahiya N, Khadka A, Sharma AK, Gupta AK, Singh N, Brashier DBS (2014) Denosumab: a bone antiresorptive drug. Medical Journal Armed Forces India 71(1):71–75
Reid IR (2011) Bisphosphonates in the treatment of osteoporosis: a review of their contribution and controversies. Skelet Radiol 40(9):1191–1196
Riggs BL, Hartmann LC (2003) Selective estrogen-receptor modulators mechanisms of action and application to clinical practice. New Engl J Med 348(7):618–629
Muñoztorres M, Alonso G, Raya MP (2004) Calcitonin therapy in osteoporosis. Treat Endocrinol 3(2):117–132
Hajime M, Okada Y, Mori H, Tanaka Y (2014) A case of teriparatide-induced severe hypophosphatemia and hypercalcemia. J Bone Miner Metab 32(5):601–604
Oryan A, Kamali A, Moshiri A (2015) Potential mechanisms and applications of statins on osteogenesis: current modalities, conflicts and future directions. J Controll Release 215:12–24
Liu J, Zhu LP, Yang XL, Huang HL, Ye DQ (2013) HMG-CoA reductase inhibitors (statins) and bone mineral density: a meta-analysis. Bone 54(1):151–156
Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY (2007) Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone 40(6):1581–1587
Hatzigeorgiou C, Jackson JL (2005) Hydroxymethylglutaryl-coenzyme a reductase inhibitors and osteoporosis: a meta-analysis. Osteoporosis Int 16(8):990–998
Jin SL, Jiang JP, Bai PC, Zhang M, Tong X, Wang H et al (2015) Statin use and risk of fracture: a meta-analysis. Int J Clin Exp Med 8(5):8269–8275
Berthold HK, Unverdorben S, Zittermann A, Degenhardt R, Baumeister B, Unverdorben M et al (2004) Age-dependent effects of atorvastatin on biochemical bone turnover markers: a randomized controlled trial in postmenopausal women. Osteoporosis Int 15(6):459–467
Chen Z, Cai H, Jin X, Lu JH, Wang J, Fang NY (2014) Effects of atorvastatin on bone mineral density (BMD) and bone metabolism in elderly males with osteopenia and mild dyslipidemia: a 1-year randomized trial. Arch Gerontol Geriat 59(3):515–521
Patil S, Holt G, Raby N, Mclellan AR, Smith K, O'Kane S et al (2009) Prospective, double blind, randomized, controlled trial of simvastatin in human fracture healing. J Orthop Res 27(3):281–285
Rosenson RS, Tangney CC, Langman CB, Parker TS, Levine DM, Gordon BR (2005) Short-term reduction in bone markers with high-dose simvastatin. Osteoporosis Int 16(10):1272–1276
Rejnmark L, Buus HN, Vestergaard P, Heickendorff L, Andreasen F, Larsen LM et al (2004) Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res 19(5):737–744
Hsia J, Morse M, Levin V (2002) Effect of simvastatin on bone markers in osteopenic women: a placebo-controlled, dose-ranging trial. BMC Musculoskel Dis 3(1):1–5
Jiang J, Boyle LJ, Mikus CR, Oberlin DJ, Fletcher JA, Thyfault JP et al (2014) The effects of improved metabolic risk factors on bone turnover markers after 12 weeks of simvastatin treatment with or without exercise. Metabolism 63(11):1398–1408
Mundy GR (2007) Osteoporosis and inflammation. Nutr Rev 65(12):147–151
Reid IR, Hague W, Emberson J, Baker J, Tonkin A, Hunt D et al (2001) Effect of pravastatin on frequency of fracture in the LIPID study: secondly analysis of a randomised controlled trial. Lancet 357(9255):509–512
Pena JM, Aspberg S, Macfadyen J, Glynn RJ, Solomon DH, Ridker PM (2015) Statin therapy and risk of fracture: results from the Jupiter randomized clinical trial. Jama Inter Med 175(2):171–177
Chuengsamarn S, Rattanamongkoulgul S, Suwanwalaikorn S, Wattanasirichaigoon S, Kaufman L (2010) Effects of statins vs. non-statin lipid-lowering therapy on bone formation and bone mineral density biomarkers in patients with hyperlipidemia. Bone 46(4):1011–1015
Savić T, Janković D, Janković I, Bojanić V, Đinđić B, Miladinović-Tasić N (2010) Effects of simvastatin therapy on bone mineral density in hypercholesterolemic postmenopausal woman. Acta Facultatis Medicae Naissensis 27(1):13–18
Safaei H, Janghorbani M, Aminorroaya A, Amini M (2007) Lovastatin effects on bone mineral density in postmenopausal women with type 2 diabetes mellitus. Acta Diabetol 44(2):76–82
Lupattelli G, Scarponi AM, Vaudo G, Siepi D, Roscini AR, Gemelli F et al (2004) Simvastatin increase bone mineral density in hypercholesterolemic postmenopausal women. Metabolism 53(6):744–748
Chung YS, Lee MD, Lee SK, Kim HM, Fitzpatrick LA (2000) HMG-CoA reductase inhibitiors increase BMD in type 2 diabetes mellitus patients. J Clin Endocr Metab 85(3):1137–1142
Funkhouser HL, Adera T, Adler RA (2002) Effect of HMG-CoA reductase inhibitors (statins) on bone mineral density. J Clin Densitom 5(2):151–158
Montagnani A, Gonneli S, Cepollaro C, Pacini S, Campagna MS, Franci MB et al (2003) Effect of simvasatatin treatment on bone mineral density and bone turnover in hypercholesterolemic postempausal women: a 1-year longitudinal study. Bone 32(4):427–433
LaCroix AZ, Cauley JA, Pettinger M, Hsia J, Bauer DC, McGowan J et al (2003) Statin use, clinical fracture, and bone density in postmenopausal women: results from the women’s health initiative observation study. Ann Intern Med 139(2):97–104
Masafumi K, Yusuke S, Toshinobu A, Teruhiko K, Shigeru K, Akira N et al (2003) Atorvastatin, 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, reduces bone resorption in the elderly. J Am Geriatr Soc 51(11):1677–1678
Majima T, Shimatsu A, Komatsu Y, Satoh N, Fukao A, Ninomiya K et al (2007) Short-term effects of pitavastatin on biochemical markers of bone turnover in patients with hypercholesterolemia. Internal Med 46(24):1967–1974
Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S (2008) Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Brit J Clin Pharmaco 67(1):99–109
Scranton RE, Young M, Lawler E (2005) Statin use and fracture risk. Arch Int Med 165:2007–2012
Helin-Salmivaara A, Korhonen MJ, Lehenkari P, Junnila SY, Neuvonen PJ, Ruokoniemi P et al (2012) Statins and hip fracture prevention—a population based cohort study in women. PLoS One 7(10):e48095
Schoofs MW, Sturkenboom MC, Klift M, Hofman A, Pols HA, Stricker BH (2004) HMG-CoA reductase inhibitors and the risk of vertebral fracture. J Bone Miner Res 19(9):1525–1529
Ray WA, Daugherty JR, Griffin MR (2002) Lipid-lowering agents and the risk of hip fracture in a medicaid population. Injury Prev 8(4):276–279
Ward IM, Mortensen EM, Battafarano DF, Frei CR, Mansi I (2014) Association of statins and risk of fractures in a military health system: a propensity score-matched analysis. Annals of Pharamacotherapy 48(11):1406–1411
Staa TP, Wegman S, De VF, Leufkens B, Cooper C (2001) Use of statins and risk of fractures. Jama-J Am Med Assoc 285(14):1850–1856
Rejnmark L, Olsen ML, Johnsen SP, Vestergaard P, Sorensen ST, Mosekilde L (2004) Hip fracture risk in statin users—a population-based Danish case-control study. Osteoporosis Int 15(6):452–458
Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H (2000) HMG-CoA reductase inhibitors and the risk of fractures. Jama-J Am Med Assoc 283(24):3205–3210
Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG et al (2000) Inhibitors of hydroxymethylglutaryl-coenzyme a reductase and risk of fracture among older women. Lancet 355(9222):2185–2188
Bakhireva LN, Shainline MR, Carter S, Robinson S, Beaton SJ, Nawarskas JJ et al (2010) Synergistic effect of stains and postmenopausal hormone therapy in the prevention of skeletal fractures in elderly women. Pharmacotherapy 30(9):779–887
Wang PS, Solomon DH, Mogun H, Avorn J (2000) HMG-CoA reductase inhibitors and the risk of hip fractures in elederly patients. Jama-J Am Med Assoc 283(24):3211–3216
Rejnmark L, Vestergaard P, Mosekilde L (2006) Statin but not non-statin lipid-lowering drugs decrease fracture risk: a nation-wide case-control study. Calcified Tissue Int 79(1):27–36
Ruan F, Zheng Q, Wang J (2012) Mechanisms of bone anabolism regulated by statins. Bioscience Rep 32(6):511–519
Jensen ED, Rajaram G, Westendorf JJ (2010) Regulation of gene express in osteoblasts. Biofactors 36(1):25–32
Martin JW, Zielenska M, Stein GS, Wijnen AJ, Squire JA (2011) The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma 2011(13):1–13
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343(6257):425–430
Weivoda MM, Hohl RJ (2011) Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation. Endocrinology 152(8):3113–3122
Centrella M, Mccarthy TL, Canalis E (1987) Transforming growth-factor-β is a bifunctional regulator of replication and collagen-synthesis in osteoblast-enriched cell-cultures from fetal-rat bone. J Biol Chem 262(6):2869–2874
Heldin CH, Miyazono K, Dijke PT (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390(6659):465–471
Tsubaki M, Satou T, Itoh T, Imano M, Yanae M, Kato C et al (2012) Bisphosphonate-and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol Cell Endocrinol 361(2):219–231
Wishart J, Horowitz M, Need A, Nordin BE (1990) Relationship between forearm and vertebral mineral density in postmenopausal women with primary hyperparathyroidism. Arch Intern Med 150(6):1329–1331
Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359(9320):1841–1850
Hamelin BA, Turgeon J (1998) Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci 19(1):26–37
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G et al (1999) Stimulation of bone formation in vitro and in rodents by statins. Science 286(5446):1946–1949
Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE et al (2004) Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med 164(2):146–152
Pasco JA, Kotowicz MA, Henry MJ, Sanders KM, Nicholson GC (2002) Statin use, bone mineral density, and fracture risk: Geelong osteoporosis study. Arch Int Med 162(5):537–540
Johansson H, Kanis JA, Odén A, McCloskey E, Chapurlat RD, Christiansen C et al (2014) A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 29(1):223–233
Ivers RQ, Cumming RG, Mitchell P, Peduto AJ (2001) Diabetes and risk of fracture: the blue mountains eye study. Diabetes Care 24(7):1198–1203
Grasser WA, Baumann AP, Petras SF, Harwood HJ Jr, Devalaraja R, Renkiewicz R et al (2003) Regulation of osteoclast differentiation by statins. J Musculoskel Neuron 3(1):53–62
Acknowledgements
This project was supported by grants from the “Faculty of Medical Devices-Jingxin” Cooperation Fund of Shenyang Pharmaceutical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
.
Supplementary Table 1
(DOCX 13 kb)
.
Supplementary Table 2
(DOCX 15 kb)
.
Supplementary Table 3
(DOCX 13 kb)
.
Supplementary Table 4
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
An, T., Hao, J., Sun, S. et al. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int 28, 47–57 (2017). https://doi.org/10.1007/s00198-016-3844-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3844-8