Abstract
Summary
Oxygen ultra-fine bubbles (OUB) saline injection prevents bone loss of glucocorti\coid-induced osteoporosis in mice, and OUB inhibit osteoclastogenesis via RANK-TRAF6-c-Fos-NFATc1 signaling and RANK-p38 MAPK signaling in vitro.
Introduction
Ultra-fine bubbles (<200 nm in diameter) have several unique properties, and they are tested in various medical fields. The purpose of this study was to investigate the effects of oxygen ultra-fine bubbles (OUB) on glucocorticoid-induced osteoporosis (GIO) model mice.
Methods
Prednisolone (PSL, 5 mg) was subcutaneously inserted in 6-month-old male C57BL/6J mice, and 200 μl of saline, OUB-diluted saline, or nitrogen ultra-fine bubbles (NUB)-diluted saline was intraperitoneally injected three times per week for 8 weeks the day after operations. Mice were divided into four groups; (1) control, sham-operation + saline; (2) GIO, PSL + saline; (3) GIO + OUB, PSL + OUB saline; (4) GIO + NUB, PSL + NUB saline. The effects of OUB on osteoblasts and osteoclasts were examined by serially diluted OUB medium in vitro.
Results
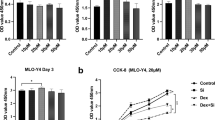
Bone mass was significantly decreased in GIO [bone volume/total volume (%): control vs. GIO 12.6 vs. 7.9; p < 0.01] while significantly preserved in GIO + OUB (GIO vs. GIO + OUB 7.9 vs. 12.9; p < 0.05). In addition, tartrate-resistant acid phosphatase (TRAP)-positive cells in the distal femur [mean osteoclasts number/bone surface (mm−1)] was significantly increased in GIO (control vs. GIO 6.8 vs. 11.6; p < 0.01) while suppressed in GIO + OUB (GIO vs. GIO + OUB 11.6 vs. 7.5; p < 0.01). NUB did not affect these parameters. In vitro experiments revealed that OUB significantly inhibited osteoclastogenesis by inhibiting RANK-TRAF6-c-Fos-NFATc1 signaling, RANK-p38 MAPK signaling, and TRAP/Cathepsin K/DC-STAMP mRNA expression in a concentration-dependent manner. OUB did not affect osteoblastogenesis in vitro.
Conclusions
OUB prevent bone loss in GIO mice by inhibiting osteoclastogenesis.






Similar content being viewed by others
References
Agarwal A, Ng WJ, Liu Y (2011) Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84:1175–1180
Ebina K, Shi K, Hirao M, Hashimoto J, Kawato Y, Kaneshiro S, Morimoto T, Koizumi K, Yoshikawa H (2013) Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS One 8:e65339
Takahashi M, Chiba K, Li P (2007) Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J Phys Chem B 111:1343–1347
Matsuki N, Ichiba S, Ishikawa T, Nagano O, Takeda M, Ujike Y, Yamaguchi T (2012) Blood oxygenation using microbubble suspensions. European biophysics journal: EBJ 41:571–578
Bitterman H (2009) Bench-to-bedside review: oxygen as a drug. Crit Care 13:205
Abdelsalam M, Cheifetz IM (2010) Goal-directed therapy for severely hypoxic patients with acute respiratory distress syndrome: permissive hypoxemia. Respir Care 55:1483–1490
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89:219–229
Wang Y, Li X, Zhou Y, Huang P, Xu Y (2010) Preparation of nanobubbles for ultrasound imaging and intracellular drug delivery. Int J Pharm 384:148–153
Hirose Y, Yasui T, Taguchi K et al (2013) Oxygen nano-bubble water reduces calcium oxalate deposits and tubular cell injury in ethylene glycol-treated rat kidney. Urolithiasis 41:279–294
Al Hadi H, Smerdon GR, Fox SW (2013) Hyperbaric oxygen therapy suppresses osteoclast formation and bone resorption. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 31:1839–1844
Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR (2006) Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 312:1693–1702
Arnett TR (2010) Acidosis, hypoxia and bone. Arch Biochem Biophys 503:103–109
Utting JC, Flanagan AM, Brandao-Burch A, Orriss IR, Arnett TR (2010) Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct 28:374–380
Harrison JS, Rameshwar P, Chang V, Bandari P (2002) Oxygen saturation in the bone marrow of healthy volunteers. Blood 99:394
Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 66:889–900
Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48:3224–3229
Rizzoli R, Adachi JD, Cooper C et al (2012) Management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 91:225–243
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 13:777–787
Toth M, Grossman A (2013) Glucocorticoid-induced osteoporosis: lessons from Cushing’s syndrome. Clin Endocrinol 79:1–11
Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A (2006) Glucocorticoid-induced osteoporosis: an update. Trends in endocrinology and metabolism: TEM 17:144–149
Suzuki Y, Nawata H, Soen S et al (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350
Khan AA, Morrison A, Hanley DA et al (2015) Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res Off J Am Soc Bone Miner Res 30:3–23
Yoon HY, Won YY, Chung YS (2012) Poncirin prevents bone loss in glucocorticoid-induced osteoporosis in vivo and in vitro. J Bone Miner Metab 30:509–516
Kawai S, Takagi Y, Kaneko S, Kurosawa T (2011) Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Experimental animals/Japanese Association for Laboratory Animal Science 60:481–487
Noguchi T, Ebina K, Hirao M et al (2015) Progranulin plays crucial roles in preserving bone mass by inhibiting TNF-alpha-induced osteoclastogenesis and promoting osteoblastic differentiation in mice. Biochem Biophys Res Commun 465:638–643
He Y, Rhodes SD, Chen S et al (2012) C-Fms signaling mediates neurofibromatosis type-1 osteoclast gain-in-functions. PLoS One 7 e46900
Hu M, Bassett JH, Danks L et al (2011) Activated invariant NKT cells regulate osteoclast development and function. J Immunol 186:2910–2917
Schmidt K, Schinke T, Haberland M, Priemel M, Schilling AF, Mueldner C, Rueger JM, Sock E, Wegner M, Amling M (2005) The high mobility group transcription factor Sox8 is a negative regulator of osteoblast differentiation. J Cell Biol 168:899–910
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM (2003) Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 111:821–831
Imura Y, Yasui H, Outani H et al (2014) Combined targeting of mTOR and c-MET signaling pathways for effective management of epithelioid sarcoma. Mol Cancer 13:185
Peng HH, Martel J, Lee YH, Ojcius DM, Young JD (2011) Serum-derived nanoparticles: de novo generation and growth in vitro, and internalization by mammalian cells in culture. Nanomedicine (Lond) 6:643–658
Xie X, Lin W, Liu H, Deng J, Chen Y, Liu H, Fu X, Yang Y (2015) Ultrasound-responsive nanobubbles contained with peptide-camptothecin conjugates for targeted drug delivery. Drug delivery 1–9
Huang HY, Liu HL, Hsu PH, Chiang CS, Tsai CH, Chi HS, Chen SY, Chen YY (2015) A multitheragnostic nanobubble system to induce blood-brain barrier disruption with magnetically guided focused ultrasound. Adv Mater 27:655–661
Geis NA, Katus HA, Bekeredjian R (2012) Microbubbles as a vehicle for gene and drug delivery: current clinical implications and future perspectives. Curr Pharm Des 18:2166–2183
Dayton PA, Chomas JE, Lum AF, Allen JS, Lindner JR, Simon SI, Ferrara KW (2001) Optical and acoustical dynamics of microbubble contrast agents inside neutrophils. Biophys J 80:1547–1556
Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K (2000) Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation 101:668–675
Chiou WF, Huang YL, Liu YW (2014) (+)-Vitisin a inhibits osteoclast differentiation by preventing TRAF6 ubiquitination and TRAF6-TAK1 formation to suppress NFATc1 activation. PLoS One 9 e89159
Irwin R, LaPres JJ, Kinser S, McCabe LR (2007) Prolyl-hydroxylase inhibition and HIF activation in osteoblasts promotes an adipocytic phenotype. J Cell Biochem 100:762–772
Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S (2003) Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol 196:2–8
Hamilton JA, Lacey DC, Turner A, de Kok B, Huynh J, Scholz GM (2012) Hypoxia enhances the proliferative response of macrophages to CSF-1 and their pro-survival response to TNF. PLoS One 7:e45853
Hsieh CP, Chiou YL, Lin CY (2010) Hyperbaric oxygen-stimulated proliferation and growth of osteoblasts may be mediated through the FGF-2/MEK/ERK 1/2/NF-kappaB and PKC/JNK pathways. Connect Tissue Res 51:497–509
Zahm AM, Bucaro MA, Srinivas V, Shapiro IM, Adams CS (2008) Oxygen tension regulates preosteocyte maturation and mineralization. Bone 43:25–31
Tuncay OC, Ho D, Barker MK (1994) Oxygen tension regulates osteoblast function. American journal of orthodontics and dentofacial orthopedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 105:457–463
Acknowledgements
We are grateful to M. Shinkawa and A. Tada for their excellent technical assistance. We thank all the members of Yoshikawa’s laboratory for the helpful discussion and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All experimental protocols were approved by the Ethics Review Committee for Animal Experimentation of Osaka University Graduate School of Medicine (permission number 24-022-007).
Authors’ roles
Study design: TN, KE, MH, and HY. Study conduct: TN and KE. Data collection: TN, KE, MH, TM, KK, KK, HM, and TI. Data analysis: TN and KE. Data interpretation: TN, KE, and MH. Drafting the manuscript: TN and KE. Approving the final version of manuscript: TN, KE, MH, TM, KK, KK, HM, TI, and HY. KE takes responsibility for the integrity of the data analysis.
Funding
All oxygen and nitrogen ultra-fine bubbles-diluted saline and medium were prepared by Ligaric Company Limited. This research was funded by Nakatomi funding, Health and Labor Sciences Research Grant of Japan, Ligaric Company Limited, and West Nippon Expressway Company. The funders had no role in the study design, decision to publish, or preparation of the manuscript.
Conflict of interest
Osaka University and West Nippon Expressway Company are applying for a patent of the medical use of ultra-fine bubbles.
Electronic supplementary material
ESM 1
(PDF 304 kb)
Rights and permissions
About this article
Cite this article
Noguchi, T., Ebina, K., Hirao, M. et al. Oxygen ultra-fine bubbles water administration prevents bone loss of glucocorticoid-induced osteoporosis in mice by suppressing osteoclast differentiation. Osteoporos Int 28, 1063–1075 (2017). https://doi.org/10.1007/s00198-016-3830-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3830-1