Abstract
Summary
Two strains of mice with distinct bone morphologies and mechanical properties were treated with zoledronate. Our results show a different response to drug treatment in the two strains providing evidence that baseline properties of structure/material may influence response to zoledronate.
Introduction
Bisphosphonates are highly effective in reducing fracture risk, yet some individuals treated with these agents still experience fracture. The goal of this study was to test the hypothesis that genotype influences the effect of zoledronate on bone mechanical properties.
Methods
Skeletally mature male mice from genetic backgrounds known to have distinct baseline post-yield properties (C57/B6, high post-yield displacement; A/J, low post-yield displacement) were treated for 8 weeks with saline (VEH) or zoledronate (ZOL, 0.06 mg/kg subcutaneously once every 4 weeks) in a 2 × 2 study design. Ex vivo μCT and mechanical testing (4-pt bending) were conducted on the femur to assess morphological and mechanical differences.
Results
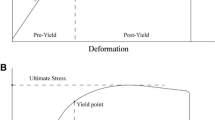
Significant drug and/or genotype effects were found for several mechanical properties and significant drug × genotype interactions were found for measures of strength (ultimate force) and brittleness (total displacement, strain to failure). Treatment with ZOL affected bone biomechanical measures of brittleness (total displacement (−25 %) and strain to failure (−23 %)) in B6 mice significantly differently than in A/J mice. This was driven by unique drug × genotype effects on bone geometry in B6 animals yet likely also reflected changes to the tissue properties.
Conclusion
These data may support the concept that properties of the bone geometry and/or tissue at the time of treatment initiation play a role in determining the bone’s mechanical response to zoledronate treatment.



Similar content being viewed by others
References
Reid IR (2015) Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol 11:418–28
Lewiecki EM (2003) Nonresponders to osteoporosis therapy. J Clin Densitom 6:307–314
Chapurlat RD, Cummings SR (2002) Does follow-up of osteoporotic women treated with antiresorptive therapies improve effectiveness? Osteoporos Int 13:738–744
Sebba AI, Bonnick SL, Kagan R et al (2004) Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosis. Curr Med Res Opin 20:2031–2041
Nakano T, Yamamoto M, Hashimoto J et al (2015) Higher response with bone mineral density increase with monthly injectable ibandronate 1 mg compared with oral risedronate in the MOVER study. J Bone Miner Metab. doi:10.1007/s00774-015-0717-8
Carmel AS, Shieh A, Bang H, Bockman RS (2012) The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml. Osteoporos Int 23:2479–2487
Baxter I, Rogers A, Eastell R, Peel N (2012) Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos Int 24:941–947
Obermayer-Pietsch BM, Marín F, Mccloskey EV et al (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600
Goldman HM, Hampson NA, Guth JJ et al (2014) Intracortical remodeling parameters are associated with measures of bone robustness. Anat Rec (Hoboken) 297:1817–1828. doi:10.1002/ar.22962
Jepsen KJ, Centi A, Duarte GF et al (2011) Biological constraints that limit compensation of a common skeletal trait variant lead to inequivalence of tibial function among healthy young adults. J Bone Miner Res 26:2872–2885
Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH (2003) Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome 14:97–104
Price C, Herman BC, Lufkin T et al (2005) Genetic variation in bone growth patterns defines adult mouse bone fragility. J Bone Miner Res 20:1983–1991
Jepsen KJ, Hu B, Tommasini SM et al (2007) Genetic randomization reveals functional relationships among morphologic and tissue-quality traits that contribute to bone strength and fragility. Mamm Genome 18:492–507
Allen MR, Burr DB (2011) Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone 49:56–65
Kubek D, Burr D, Allen M (2010) Ovariectomy stimulates and bisphosphonates inhibit intracortical remodeling in the mouse mandible. Orthod Craniofac Res 13:214–222
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Jepsen KJ, Silva MJ, Vashishth D et al (2015) Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J Bone Miner Res 30:951–66
Wallace JM, Burr DB, Allen MR (2014) Chapter 6—skeletal hard tissue biomechanics. In: Basic and applied bone biology. Academic Press, San Diego, pp 115–130
Berman AG, Wallace JM, Bart ZR, Allen MR (2015) Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biol 52–54:19–28
Black DM, Delmas PD, Eastell R, Reid IR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031
Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ (2005) Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res 20:1372–1380
Acevedo C, Bale H, Gludovatz B et al (2015) Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone 81:352–363. doi:10.1016/j.bone.2015.08.002
Burr DB, Liu Z, Allen MR (2015) Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 71:58–62
Currey JD (2003) Role of collagen and other organics in the mechanical properties of bone. Osteoporos Int 14(Suppl 5):S29–36
Reilly GC, Currey JD (2000) The effects of damage and microcracking on the impact strength of bone. J Biomech 33:337–343
Vashishth D, Gibson GJ, Khoury JI et al (2001) Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28:195–201
Granke M, Does MD, Nyman JS (2015) The role of water compartments in the material properties of cortical bone. Calcif Tissue Int 97:292–307
Acknowledgments
This work was supported by NIH grants AR62002 (MRA), DK108554 (F32 support for EM), and AR65971 (T32 support for MA). The microCT utilized in this experiment was purchased through a NIH S10 grant (OD 016208).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Prior to initiating these studies, all procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Aref, M.W., McNerny, E.M.B., Brown, D. et al. Zoledronate treatment has different effects in mouse strains with contrasting baseline bone mechanical phenotypes. Osteoporos Int 27, 3637–3643 (2016). https://doi.org/10.1007/s00198-016-3701-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3701-9