Abstract
Summary
Precision errors of cortical bone micro-architecture from high-resolution peripheral quantitative computed tomography (pQCT) ranged from 1 to 16 % and did not differ between automatic or manually modified endocortical contour methods in postmenopausal women or young adults. In postmenopausal women, manually modified contours led to generally higher cortical bone properties when compared to the automated method.
Introduction
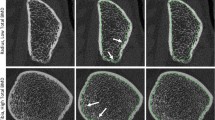
First, the objective of the study was to define in vivo precision errors (coefficient of variation root mean square (CV%RMS)) and least significant change (LSC) for cortical bone micro-architecture using two endocortical contouring methods: automatic (AUTO) and manually modified (MOD) in two groups (postmenopausal women and young adults) from high-resolution pQCT (HR-pQCT) scans. Second, it was to compare precision errors and bone outcomes obtained with both methods within and between groups.
Methods
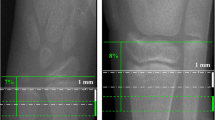
Using HR-pQCT, we scanned twice the distal radius and tibia of 34 postmenopausal women (mean age ± SD 74 ± 7 years) and 30 young adults (27 ± 9 years). Cortical micro-architecture was determined using AUTO and MOD contour methods. CV%RMS and LSC were calculated. Repeated measures and multivariate ANOVA were used to compare mean CV% and bone outcomes between the methods within and between the groups. Significance was accepted at P < 0.05.
Results
CV%RMS ranged from 0.9 to 16.3 %. Within-group precision did not differ between evaluation methods. Compared to young adults, postmenopausal women had better precision for radial cortical porosity (precision difference 9.3 %) and pore volume (7.5 %) with MOD. Young adults had better precision for cortical thickness (0.8 %, MOD) and tibial cortical density (0.2 %, AUTO). In postmenopausal women, MOD resulted in 0.2–54 % higher values for most cortical outcomes, as well as 6–8 % lower radial and tibial cortical BMD and 2 % lower tibial cortical thickness.
Conclusions
Results suggest that AUTO and MOD endocortical contour methods provide comparable repeatability. In postmenopausal women, manual modification of endocortical contours led to generally higher cortical bone properties when compared to the automated method, while no between-method differences were observed in young adults.


Similar content being viewed by others
References
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22(3):465–475
Riggs BL, Melton LJ 3rd (1995) The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17(5):505S–511S
Bouillon R, Burckhardt P, Christiansen C, Fleisch H, Fujita T, Gennari C, Martin TJ, Mazzuoli G, Melton LJ, Ringe JD, Riis B, Peck WA, Samsioe G, Shulman LE (1991) Consensus development conference: prophylaxis and treatment of osteoporosis. Osteoporos Int 1:114–117
Mueller TL, van Lenthe GH, Stauber M, Gratzke C, Eckstein F, Muller R (2009) Regional, age and gender differences in architectural measures of bone quality and their correlation to bone mechanical competence in the human radius of an elderly population. Bone 45(5):882–891
Spadaro JA, Werner FW, Brenner RA, Fortino MD, Fay LA, Edwards WT (1994) Cortical and trabecular bone contribute strength to the osteopenic distal radius. J Orthop Res 12(2):211–218
Alffram P-A, Bauer GCH (1962) Epidemiology of fractures of the forearm: a biomechanical investigation of bone strength. J Bone Joint Surg 44A(1):105–114
Owen RA, Melton L Jr, Johnson KA, Ilstrup DM, Riggs BL (1982) Incidence of Colles’ fracture in a North American community. Am J Public Health 72(6):605–607
Augat P, Claes LE (1996) Prediction of fracture load at differenet skeletal sites by geometric properties of the cortical shell. J Bone Miner Res 11(9):1356–1363
MacNeil JA, Boyd SK (2007) Load distribution and the predictive power of morphological indices in the distal radius and tibia by high resolution peripheral quantitative computed tomography. Bone 41(1):129–137
Augat P, Schorlemmer S (2006) The role of cortical bone and its microstructure in bone strength. Age Ageing 35(Suppl 2):ii27–ii31
Currey JD (1979) Changes in the impact energy absorption of bone with age. J Biomech 12:459–469
Currey JD (1988) The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech 21(2):131–139
Currey J (2004) Incompatible mechanical properties in compact bone. J Theor Biol 231(4):569–580
McCalden RW, McGeough JA, Barker MB, Court-Brown CM (1993) Age related changes in the tensile properties of cortical bone. J Bone Joint Surg 75A(8):1193–1205
Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM (2013) Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res 28(2):313–324
Bell KL, Loveridge N, Jordan GR, Power CR (2000) A novel mechanism for induction of increased cortical porosity in cases of intracapsular hip fracture. Bone 26:305–313
Bala Y, Zebaze R, Ghasem-Zadeh A, Atkinson EJ, Iuliano S, Peterson JM, Amin S, Bjornerem A, Melton LJ 3rd, Johansson H, Kanis JA, Khosla S, Seeman E (2014) Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res 29(6):1356–1362
Bell KL, Loveridge N, Power J, Garrahan N, Meggitt BF, Reeve J (1999) Regional differences in cortical porosity in the fractured femoral neck. Bone 24(1):57–64
Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK (2010) Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone 47(3):519–528
Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK (2010) Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res 25(4):882–890
Ostertag A, Peyrin F, Fernandez S, Laredo JD, de Vernejoul MC, Chappard C (2014) Cortical measurements of the tibia from high resolution peripheral quantitative computed tomography images: a comparison with synchrotron radiation micro-computed tomography. Bone 63C:7–14
Kawalilak CE, Johnston JD, Olszynski WP, Leswick D, Kontulainen SA (2014) Comparison of short term in vivo precision of bone density and micro-architecture at the distal radius and tibia between postmenopausal women and young adults. J Clin Densitom 17(4):510–517
Kreiger N, Tenehouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP, Prior JC, Rittmaster RS (1999) The Canadian multicentre osteoporosis study (CaMos): background, rationale, methods. Can J Aging 18(3):376–387
Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM, Kvern B, Siminoski K, Leslie WD, Scientific Advisory Council of Osteoporosis C (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Can Med Assoc J 182(17):1864–1873
Medical S (2011) Xtreme CT operations manual, Version 61st edn. SCANCO Medical Ag Fabrikweg 2 CH-8306, Bruettisellen, Switzerland
Kawalilak CE, Johnston JD, Olszynski WP, Kontulainen SA (2014) Characterizing micro-architectural changes at the distal radius and tibia in postmenopausal women using HR-pQCT. Osteoporos Int 25(8):2057–2066
Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S (2012) Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone 50(1):111–118
Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK (2007) Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41(4):505–515
Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK (1995) Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 5:262–270
Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM (2008) Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom 11(1):123–162
Gluer CC (1999) Monitoring skeletal changes by radiological techniques. J Bone Miner Res 14(11):1952–1962
Bonnick SL, Johnston CC, Kleerekoper M, Lindsay R, Miller P, Sherwood L, Siris E (2001) Importance of precision in bone density measurements. J Clin Densitom 4(2):105–110
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res 2(3):595–610
Zebaze RMD, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375:1729–1736
Jorgenson BL, Buie HR, McErlain DD, Sandino C, Boyd SK (2015) A comparison of methods for in vivo assessment of cortical porosity in the human appendicular skeleton. Bone 73:167–175
Swinford RR, Warden SJ (2010) Factors affecting short-term precision of musculoskeletal measures using peripheral quantitative computed tomography (pQCT). Osteoporos Int 21(11):1863–1870
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42(3):467–475
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Acknowledgments
We would like to thank all our CaMos Saskatoon cohort participants for their continued altruism and volunteering for scientific research. We thank Andrew Frank and Jola Thingvold for coordinating the study measurements. The authors also acknowledge the University of Saskatchewan and Canadian Institutes of Health Research (CIHR) for their funding support. This work was supported in part by grants from CIHR Regional Partnership Program New Investigator Award, Saskatchewan Health Research Foundation, and Canadian Foundation for Innovation (CFI 16935) and a CIHR Operating Grant (MOP98002).
Conflicts of interest
Chantal E Kawalilak, James D Johnston, Wojciech Olszynski, David ML Cooper, and Saija A Kontulainen declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawalilak, C.E., Johnston, J.D., Cooper, D.M.L. et al. Role of endocortical contouring methods on precision of HR-pQCT-derived cortical micro-architecture in postmenopausal women and young adults. Osteoporos Int 27, 789–796 (2016). https://doi.org/10.1007/s00198-015-3262-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3262-3