Abstract
Summary
This study investigates the performance and correlation of sclerostin measurements by two commercially available sclerostin ELISAs from TECOmedical and Biomedica. We found that the correlation between the results of two sclerostin assays is strong.
Introduction
Circulating sclerostin levels may provide insight into the pathophysiology of metabolic bone diseases such as osteoporosis. However, recent studies suggest that commercially available assays give different results. We compare the analytical performance of the two most used commercially available sclerostin ELISAs from TECOmedical and Biomedica.
Methods
Sclerostin levels were assessed in 20 paired serum, EDTA, and heparin plasma convenience samples from hospitalized patients. In addition, sclerostin was measured in serum samples from 34 patients with metabolic bone diseases and from 10 patients with chronic kidney disease (CKD). Samples from three healthy donors were used to determine stability and intra- and inter- assay precision.
Results
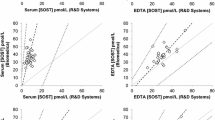
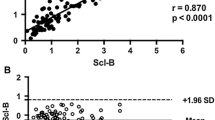
The average serum sclerostin concentration of all patients (n = 64) was 0.713 ± 0.58 ng/mL with the Biomedica assay and 0.734 ± 0.43 ng/mL with the TECO assay (p < 0.05). The results correlated strongly (r = 0.9; p < 0.0001), with Passing-Bablok regression showing a linear relationship but with a slight systematic and proportional difference between both assays. Sclerostin levels were about 30 % higher in plasma than in serum for both assays, while no significant difference was seen between EDTA and heparin plasma. Intra- and inter- precision were <10 % for TECO and <20 % for Biomedica. Samples were stable for up to three freeze-thaw cycles with both assays.
Conclusions
The two commercially available ELISAs for measuring circulating levels of sclerostin are comparable. However, given the small but statistically significant systematic and proportional differences between both assays, results and reference ranges will be assay-specific. Results will also be specific to serum or plasma.


Similar content being viewed by others
References
van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199(6):805–814. doi:10.1084/jem.20031454
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95(11):5056–5062. doi:10.1210/jc.2010-0720
Kaji H, Imanishi Y, Sugimoto T, Seino S (2011) Comparisons of serum sclerostin levels among patients with postmenopausal osteoporosis, primary hyperparathyroidism and osteomalacia. Exp Clin Endocrinol Diabetes 119(7):440–444. doi:10.1055/s-0031-1275661
Costa AG, Cremers S, Rubin MR, McMahon DJ, Sliney J Jr, Lazaretti-Castro M, Silverberg SJ, Bilezikian JP (2011) Circulating sclerostin in disorders of parathyroid gland function. J Clin Endocrinol Metab 96(12):3804–3810. doi:10.1210/jc.2011-0566
Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, Capodarca C, Franci MB, Campagna MS, Calabro A, Cataldo D, Stolakis K, Dotta F, Nuti R (2012) Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab 97(5):1737–1744. doi:10.1210/jc.2011-2958
Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, Hampson G (2012) Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int 90(6):473–480. doi:10.1007/s00223-012-9595-4
Voskaridou E, Christoulas D, Plata E, Bratengeier C, Anastasilakis AD, Komninaka V, Kaliontzi D, Gkotzamanidou M, Polyzos SA, Dimopoulou M, Terpos E (2012) High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res 44(12):909–913. doi:10.1055/s-0032-1312618
Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM (2012) Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab 97(9):E1736–E1740. doi:10.1210/jc.2012-1579
Gaudio A, Privitera F, Battaglia K, Torrisi V, Sidoti MH, Pulvirenti I, Canzonieri E, Tringali G, Fiore CE (2012) Sclerostin levels associated with inhibition of the Wnt/beta-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab 97(10):3744–3750. doi:10.1210/jc.2012-1901
Ardawi MS, Rouzi AA, Qari MH (2012) Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab 97(10):3691–3699. doi:10.1210/jc.2011-3361
Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY (2013) Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 56(2):355–362. doi:10.1016/j.bone.2013.06.029
McNulty M, Singh RJ, Li X, Bergstralh EJ, Kumar R (2011) Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays. J Clin Endocrinol Metab 96(7):E1159–E1162. doi:10.1210/jc.2011-0254
Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD (2013) Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 24(2):489–494. doi:10.1007/s00198-012-1978-x
van Lierop AH, van der Eerden AW, Hamdy NA, Hermus AR, den Heijer M, Papapoulos SE (2012) Circulating sclerostin levels are decreased in patients with endogenous hypercortisolism and increase after treatment. J Clin Endocrinol Metab 97(10):E1953–E1957. doi:10.1210/jc.2012-2218
Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R (2013) Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab 98(9):3873–3883. doi:10.1210/jc.2013-2113
Weidauer SE, Schmieder P, Beerbaum M, Schmitz W, Oschkinat H, Mueller TD (2009) NMR structure of the Wnt modulator protein Sclerostin. Biochem Biophys Res Commun 380(1):160–165. doi:10.1016/j.bbrc.2009.01.062
Veverka V, Henry AJ, Slocombe PM, Ventom A, Mulloy B, Muskett FW, Muzylak M, Greenslade K, Moore A, Zhang L, Gong J, Qian X, Paszty C, Taylor RJ, Robinson MK, Carr MD (2009) Characterization of the structural features and interactions of sclerostin: molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem 284(16):10890–10900. doi:10.1074/jbc.M807994200
Lierop AH, Hamdy NAT, Bezooijen RL, Löwik CW, Papapoulos SE (2011) The role of sclerostin in the pathophysiology of sclerosing bone dysplasias. Clin Rev Bone Miner Metab 10(2):108–116. doi:10.1007/s12018-011-9123-5
Souberbielle JC, Friedlander G, Cormier C (2006) Practical considerations in PTH testing. Clin Chim Acta 366(1–2):81–89. doi:10.1016/j.cca.2005.10.010
Acknowledgments
This work was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040. We gratefully thank Drs. Shonni Silverberg, Marcella Walker, Mishaela Rubin, Emily Stein, and Thomas Nickolas for contributing de-identified serum samples from their ongoing studies for this study.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, A.G., Cremers, S., Dworakowski, E. et al. Comparison of two commercially available ELISAs for circulating sclerostin. Osteoporos Int 25, 1547–1554 (2014). https://doi.org/10.1007/s00198-014-2635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2635-3