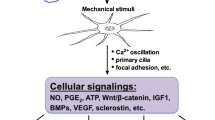
Abstract
One of the most serious healthcare problems in the world is bone loss and fractures due to a lack of physical activity in elderly people as well as in bedridden patients or otherwise inactive youth. Crucial here are the osteocytes. Buried within our bones, these cells are believed to be the mechanosensors that stimulate bone formation in the presence of mechanical stimuli and bone resorption in the absence of such stimuli. Intercellular signaling is an important physiological phenomenon involved in maintaining homeostasis in all tissues. In bone, intercellular communication via chemical signals like NO plays a critical role in the dynamic process of bone remodeling. If bones are mechanically loaded, fluid flows through minute channels in the bone matrix, resulting in shear stress on the cell membrane that activates the osteocyte. Activated osteocytes produce signaling molecules like NO, which modulate the activity of the bone-forming osteoblasts and the bone-resorbing osteoclasts, thereby orchestrating bone adaptation to mechanical loading. In this review, we highlight current insights in the role of NO in the mechanical adaptation of bone mass and structure, with emphasis on its role in local bone gain and loss as well as in remodeling supervised by osteocytes. Since mechanical stimuli and NO production enhance bone strength and fracture resistance, these new insights may facilitate the development of novel osteoporosis treatments.





Similar content being viewed by others
References
Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408–411
Fox SW, Chambers TJ, Chow JW (1996) Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Physiol 270:E955–E960
Van ‘t Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103:255–261
Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147(Suppl 1):S193–S201
Zhou L, Zhu DY (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20:223–230
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, Platts LA, Hukkanen M, Polak JM, Lanyon LE (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 14:1123–1131
Hukkanen MV, Platts LA, Fernandez DM, O’Shaughnessy M, MacIntyre I, Polak JM (1999) Developmental regulation of nitric oxide synthase expression in rat skeletal bone. J Bone Miner Res 14:868–877
Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH (1997) Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res 12:1108–1115
Loveridge N, Fletcher S, Power J, Caballero-Alias AM, Das-Gupta V, Rushton N, Parker M, Reeve J, Pitsillides AA (2002) Patterns of osteocytic endothelial nitric oxide synthase expression in the femoral neck cortex: differences between cases of intracapsular hip fracture and controls. Bone 30:866–871
Lirani-Galvão AP, Lazaretti-Castro M, Portero-Muzy N, Bergamaschi CT, Silva OL, Carvalho AB, Delmas PD, Chavassieux P (2010) Is nitric oxide a mediator of the effects of low-intensity electrical stimulation of bone in ovariectomized rats? Calcif Tissue Int 87:52–59
Zweier JL, Wang P, Samouilov A, Kuppusamy P (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1:804–809
Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R (2000) Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275:7757–7763
Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15:391–404
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. New Engl J Med 329:2002–2012
Van ‘t Hof RJ, Armour KJ, Smith LM, Armour KE, Wei XQ, Liew FY, Ralston SH (2000) Requirement of the inducible nitrix oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proc Natl Acad Sci U S A 97:7993–7998
Armour KE, Armour KJ, Gallagher ME, Gödecke A, Helfrich MH, Reid DM, Ralston SH (2001) Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142:760–766
Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez DM, Hukkanen M, Huang P, MacIntyre I, Polak J (2001) Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol 158:247–257
Van ‘t Hof RJ, MacPhee J, Libouban H, Helfrich MH, Ralston SH (2004) Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology 145:5068–5074
Cuzzocrea S, Mazzon E, Dugo L, Genovese T, Di PR, Ruggeri Z, Vegeto E, Caputi AP, Van De Loo FA, Puzzolo D, Maggi A (2003) Inducible nitric oxide synthase mediates bone loss in ovariectomized mice. Endocrinology 144:1098–1107
Watanuki M, Sakai A, Sakata T, Tsurukami H, Miwa M, Uchida Y, Watanabe K, Ikeda K, Nakamura T (2002) Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J Bone Miner Res 17:1015–1025
Sabanai K, Tsusui M, Sakai A, Hirasawa H, Tanaka S, Nakamura E, Tanimoto A, Sasaguri Y, Ito M, Shimokawa H, Nakamura T, Yanagihara N (2008) Genetic disruption of all NO synthase isoforms enhances BMD and bone turnover in mice in vivo: involvement of the renin-angiotensin system. J Bone Miner Res 23:633–643
Wimalawansa SJ (2007) Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Ann NY Acad Sci 1117:283–297
Skerry TM (2008) The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 473:117–123
Kamioka H, Kameo Y, Imai Y, Bakker AD, Bacabac RG, Yamada N, Takaoka A, Yamashiro T, Adachi T, Klein-Nulend J (2012) Microscale fluid flow analysis in a human osteocyte canaliculus using a realistic high-resolution image-based three-dimensional model. Integr Biol 4:1198–1206
Burger EH, Klein-Nulend J (1999) Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J 13:S101–S112
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH (1995) Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J 9:441–445
Scheiner S, Pivonka P, Hellmich C (2013) Poromechanical stimulation of bone remodeling: a continuum micromechanics-based mathematical model and experimental validation. Poromechanics V:1867–1876
Turner CH, Owan I, Takano Y (1995) Mechanotransduction in bone: role of strain rate. Am J Physiol 269:E438–E442
Fritton SP, McLeod KJ, Rubin CT (2000) Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech 33:317–325
De Jong WC, Korfage JA, Langenbach GE (2010) Variations in habitual bone strains in vivo: long bone versus mandible. J Struct Biol 172:311–318
Nordstrom P, Pettersson U, Lorentzon R (1998) Type of physical activity, muscle strength, and pubertal stage as determinants of bone mineral density and bone area in adolescent boys. J Bone Miner Res 13:1141–1148
Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, van Loon JJWA, Klein-Nulend J (2004) Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun 315:823–829
Turner CH, Owan I, Jacob DS, McClintock R, Peacock M (1997) Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone 21:487–490
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66:397–402
Bacabac RG, Smit TH, Mullender MG, van Loon JJWA, Klein-Nulend J (2005) Initial stress-kick is required for fluid shear stress-induced rate dependent activation of bone cells. Ann Biomed Eng 33:104–110
You L, Cowin SC, Schaffler MB, Weinbaum S (2001) A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech 34:1375–1386
Han Y, Cowin SC, Schaffler MB, Weinbaum S (2004) Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A 101:16689–16694
Bacabac RG, Smit TH, van Loon JJWA, Zandieh-Doulabi B, Helder MN, Klein-Nulend J (2006) Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J 20:858–864
McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ (2005) A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J 19:482–484
Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH (1998) Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun 250:108–114
Rath AL, Bonewald LF, Ling J, Jiang JX, Van Dyke ME, Nicolella DP (2010) Correlation of cell strain in single osteocytes with intracellular calcium, but not intracellular nitric oxide, in response to fluid flow. J Biomech 43:1560–1564
Bakker AD, da Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, Marcantonio RA, Cirelli JA, Klein-Nulend J (2009) Tumor necrosis factor alpha and interleukin-1beta modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthr Rheum 60:3336–3345
Sugawara Y, Ando R, Kamioka H, Ishihara Y, Murshid SA, Hashimoto K, Kataoka N, Tsujioka K, Kajiya F, Yamashiro T, Takano-Yamamoto T (2008) The alteration of a mechanical property of bone cells during the process of changing from osteoblasts to osteocytes. Bone 43:19–24
McGarry JG, Klein-Nulend J, Prendergast PJ (2005) The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun 330:341–348
Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J (2006) Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res 21:1722–1728
Bacabac RG, Mizuno D, Schmidt CF, MacKintosh FC, Van Loon JJWA, Klein-Nulend J, Smit TH (2008) Round versus flat: bone cell morphology, elasticity, and mechanosensing. J Biomech 41:1590–1598, Erratum in: J Biomech 41:2786
Chow JW, Fox SW, Lean JM, Chambers TJ (1998) Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res 13:1039–1044
Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH (1995) Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem Biophys Res Commun 217:640–648
Johnson DL, McAllister TN, Frangos JA (1996) Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol Endocrinol Metab 271:E205–E208
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605
Das-Gupta V, Williamson RA, Pitsillides AA (2012) Expression of endothelial nitric oxide synthase protein is not necessary for mechanical strain-induced nitric oxide production by cultured osteoblasts. Osteoporos Int 23:2635–2647
Bakker AD, Huesa C, Hughes A, Aspden RM, van’t Hof RJ, Klein-Nulend J, Helfrich MH (2013) Endothelial nitric oxide synthase is not essential for nitric oxide production by osteoblasts subjected to fluid shear stress in vitro. Calcif Tissue Int 92:228–239
Huang B, Chen SC, Wang DL (2009) Shear flow increases S-nitrosylation of proteins in endothelial cells. Cardiovasc Res 83:536–546
Murad F, Mittal CK, Arnold WP, Katsuki S, Kimura H (1978) Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res 9:145–158
Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, Boss GR, Pilz RB (2009) Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem 284:14796–14808
Löwik CW, Nibbering PH, van de Ruit M, Papapoulos SE (1994) Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J Clin Invest 93:1465–1472
Armour KE, Van ‘t Hoff RJ, Grabowski PS, Reid DM, Ralston SH (1999) Evidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosis. J Bone Miner Res 14:2137–2142
Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM, MacIntyre I (1995) Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci U S A 92:2954–2958
MacIntyre I, Zaidi M, Alam AS, Datta HK, Moonga BS, Lidbury PS, Hecker M, Vane JR (1991) Osteoclastic inhibition: an action of nitrix oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A 88:2936–2940
Juffer P, Jaspers RT, Lips P, Bakker AD, Klein-Nulend J (2012) Expression of muscle anabolic and metabolic factors in mechanically loaded MLO-Y4 osteocytes. Am J Physiol Endocrinol Metab 302:E389–E395
Standal T, Abildgaard N, Fagerli U-M, Stordal B, Hjertner O, Borset M, Sundan A (2007) HGF inhibits BMP-induced osteoblastogenesis: possible implications for the bone disease of multiple myeloma. Blood 109:3024–3030
Adamopoulos IE, Xia Z, Lau YS, Athanasou NA (2006) Hepatocyte growth factor can substitute for M-CSF to support osteoclastogenesis. Biochem Biophys Res Commun 350:478–483
Cheung WY, Liu C, Tonelli-Zasarsky RM, Simmons CA, You L (2011) Osteocyte apoptosis is mechanically regulated and induces angiogenesis in vitro. J Orthop Res 29:523–530
Zaman G, Saxon LK, Sunters A, Hilton H, Underhill P, Williams D, Price JS, Lanyon LE (2010) Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone 46:628–642
Furumatsu T, Shen ZN, Kawai A, Nishida K, Manabe H, Oohashi T, Inoue H, Ninomiya Y (2003) Vascular endothelial growth factor principally acts as the main angiogenic factor in the early stage of human osteoblastogenesis. J Biochem 133:633–639
Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J (2009) Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res 27:1280–1287
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ (2006) Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728
Javaheri B, Stern A, Lara N, Dallas M, Zhao H, Liu Y, Bonewald LF, Johnon ML (2013) Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. doi:10.1002/jbmr.2064, Epub ahead of print
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2005) Canonical Wnt signaling promotes osteogenesis by directly stimulating RUNX2 gene expression. J Biol Chem 280:33132–33140
Bodine PV, Billiard J, Moran RA, Ponce-de-Leon H, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS (2005) The wnt antagonist secreted-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem 96:1212–1230
Glass DA 2nd, Bialek P, Ahn JD, Starbuch M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Delgado-Calle J, Saňudo C, Bolado A, Fernández AF, Arozamena J, Pascual-Carra MA, Rodriguez-Rey JC, Fraga MF, Bonewald L, Riancho JA (2012) DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J Bone Miner Res 27:926–937
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875
Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB (1998) Intracortical remodeling in adult rat long bones after fatigue loading. Bone 23:275–281
Bronckers ALJJ, Goei SW, Luo G, Karsenty G, D’souza RN, Lyaruu DM, Burger EH (1996) DNA fragmentation during bone formation in neonatal rodents assessed by transferase-mediated end labeling. J Bone Miner Res 11:1281–1291
Verborgt O, Gibson GJ, Schaffler MB (2000) Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15:60–67
Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB (2012) Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 50:1115–1122
Parfitt AM (1994) Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 55:273–286
Kamioka H, Honjo T, Takano-Yamamoto T (2001) A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone 28:145–149
Smit TH, Burger EH (2000) Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J Bone Miner Res 15:301–307
Burger EH, Klein-Nulend J, Smit TH (2003) Strain-derived canalicular fluid flow regulates osteoclast activity in a remodeling osteon—a proposal. J Biomech 36:1453–1459
Smit TH, Burger EH, Huyghe JM (2002) A case for strain-induced fluid flow as a regulator of BMU-coupling and osteonal alignment. J Bone Miner Res 18:2021–2029
Tan SD, Kuijpers-Jagtman AM, Semeins CM, Bronckers ALJJ, Maltha JC, Von den Hoff JW, Everts V, Klein-Nulend J (2006) Fluid shear stress inhibits TNFalpha-induced osteocyte apoptosis. J Dent Res 85:905–909
Bakker AD, Klein-Nulend J, Burger EH (2004) Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun 320:1163–1168
Tan SD, Bakker AD, Semeins CM, Kuijpers-Jagtman AM, Klein-Nulend J (2008) Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem Biophys Res Commun 369:1150–1154
Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB (2012) Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem 287:978–988
Kogianni G, Mann V, Noble BS (2008) Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res 23:915–927
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 5:464–475
You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR (2008) Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 42:172–179
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J (2010) Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif Tissue Int 87:461–468
Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J (2007) Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 41:745–751
Mancini L, Moradi-Bidhendi N, Brandi ML, MacIntyre I (1998) Nitric oxide superoxide and peroxynitrite modulate osteoclast activity. Biochem Biophys Res Commun 243:785–790
Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD (2000) Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405:704–706
Van Oers RFM, Ruimerman R, Tanck E, Hilbers PAJ, Huiskes R (2008) A unified theory for osteonal and hemi-osteonal remodeling. Bone 42:250–259
Mullender MG, Huiskes R (1995) Proposal for the regulatory mechanism of Wolff’s law. J Orthop Res 13:503–512
Lancaster JR Jr (1997) A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1:18–30
Lancaster JR Jr (1996) Diffusion of free nitric oxide. Methods Enzymol 268:31–50
Guo XE, Takai E, Jiang X, Xu Q, Whitesides GM, Yardley JT, Hung CT, Chow EM, Hantschel T, Costa KD (2006) Intracellular calcium waves in bone cell networks under single cell nanoindentation. Mol Cell Biomech 3:95–107
Vatsa A, Smit TH, Klein-Nulend J (2007) Extracellular NO signalling from a mechanically stimulated osteocyte. J Biomech 40(Suppl 1):S89–95
Acknowledgments
R.F.M. van Oers was supported by a grant of the University of Amsterdam.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klein-Nulend, J., van Oers, R.F.M., Bakker, A.D. et al. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int 25, 1427–1437 (2014). https://doi.org/10.1007/s00198-013-2590-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2590-4