Abstract
Summary
The relationship between baseline Fracture Risk Assessment Tool (FRAX®) and treatment efficacy was evaluated using data from a pivotal phase 3 study. Relative risk of vertebral, nonvertebral, and all clinical fractures decreased with increasing probability of fracture for bazedoxifene (BZA) versus placebo but remained generally constant for raloxifene (RLX).
Introduction
To determine whether the FRAX® predicts osteoporosis treatment efficacy, we evaluated reductions in fracture incidence associated with BZA and RLX according to baseline fracture risk determined by FRAX® using data from a phase 3 osteoporosis treatment study.
Methods
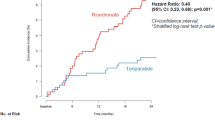
Hazard ratios (HRs) for effects of BZA and RLX versus placebo on incidence of vertebral, nonvertebral, and all clinical fractures were calculated using a Cox regression model. Cox regression analyses were performed in subgroups at or above 10-year fracture probability thresholds determined by FRAX®.
Results
HRs for the risk of vertebral, nonvertebral, and all clinical fractures versus placebo decreased with increasing 10-year fracture probability for BZA, while those for RLX remained stable. In all 10-year fracture probability subgroups, all BZA doses significantly reduced vertebral fracture risk versus placebo (HR = 0.22–0.66). BZA at 20, 40, and 20/40 mg significantly reduced risk of nonvertebral fractures (HR = 0.45, 0.44, and 0.45, respectively) and all clinical fractures (HR = 0.38, 0.41, and 0.40, respectively) for ≥20.0 % fracture probability. Vertebral fracture risk reductions for RLX 60 mg versus placebo were significant in subgroups at lower fracture probabilities (≥2.5– ≥ 10.0 %), but not higher (≥12.5 %), and in no subgroups for nonvertebral or all clinical fractures.
Conclusion
The antifracture efficacy of BZA increased with increasing baseline FRAX® score, but there was no clear relationship between RLX and baseline FRAX®. These findings provide independent confirmation of current literature, suggesting that the relationship between FRAX® and treatment efficacy varies for different agents.



Similar content being viewed by others
References
Kanis JA, on behalf of the World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary health care level. Technical report. WHO Collaborating Centre for Metabolic Bone Diseases. University of Sheffield, UK, p 288
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
World Health Organization Collaborating Centre for Metabolic Bone Diseases (2008) FRAX® WHO Fracture Risk Assessment Tool. http://www.shef.ac.uk/FRAX/. Accessed 28 October 2009
Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, Cauley JA, Compston JE, wson-Hughes B, El-Hajj FG, Johansson H, Leslie WD, Lewiecki EM, Luckey M, Oden A, Papapoulos SE, Poiana C, Rizzoli R, Wahl DA, McCloskey EV (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22:2355–2411
McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, Jalava T, Kanis JA (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy—additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20:811–817
Kanis JA, Johansson H, Oden A, McCloskey EV (2009) Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 44:1049–1054
Kanis JA, Johansson H, Oden A, McCloskey EV (2010) A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone 47:729–735
Kanis JA, Johansson H, Oden A, McCloskey EV (2011) A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX((R)). Osteoporos Int 22:2347–2355
McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, Lewiecki EM, Lorenc R, Libanati C, Kanis JA (2012) Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 27:1480–1486
Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, Woodson G, Levine AB, Constantine G, Delmas PD (2008) Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 23:525–535
Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 23:1923–1934
Pfizer (2011) CONBRIZA 20 mg film-coated tablets. Summary of product characteristics.
Pharmaceutical Business Review (2010) Pfizer introduces postmenopausal osteoporosis drug Viviant in Japan, Spain. http://drugdiscovery.pharmaceutical-business-review.com/news/pfizer-introduces-postmenopausal-osteoporosis-drug-viviant-in-japan-spain_141010. Accessed 20 January 2012
McCloskey E, Johansson H, Oden A, Chines A, Kanis J (2009) Assessment of the effect of bazedoxifene on non-vertebral fracture risk. J Bone Miner Res 24:S140, Abstract FR0376
EVISTA (raloxifene hydrochloride) tablet for oral use [package insert]. Indianapolis Eli Lilly; 2008.
European Medicines Agency (2009) European Public Assessment Report (EPAR). EVISTA. EPAR summary for the public. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000184/WC500031005.pdf. Accessed 29 July 2011
Ettinger B et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33:522–532
Donaldson MG, Palermo L, Ensrud KE, Hochberg MC, Schousboe JT, Cummings SR (2012) Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: the Fracture Intervention Trial. J Bone Miner Res 27:1804–1810
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr., Dequeker J, Favus M, for the Alendronate Phase III Osteoporosis Treatment Study Group (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ, for the Early Postmenopausal Intervention Cohort Study Group (1998) Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 338:485–492
Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP, van Staa TP, Adachi JD (2004) Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 7:255–261
Miller PD (2005) Bone density and markers of bone turnover in predicting fracture risk and how changes in these measures predict fracture risk reduction. Curr Osteoporos Rep 3:103–110
Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD (2002) Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 17:1–10
Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM (2002) Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112:281–289
Li Z, Meredith MP, Hoseyni MS (2001) A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med 20:3175–3188
Bruyere O, Detilleux J, Chines A, Reginster JY (2010) Relationships between changes in bone mineral density or bone turnover markers and vertebral fractures incidence in patients treated with bazedoxifene. Arthritis Rheum 62:S406–S407, Abstract 979
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Melton LJ III, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL (1997) Relationship of bone turnover to bone density and fractures. J Bone Miner Res 12:1083–1091
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Cummings SR, San MJ, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Acknowledgments
This study was sponsored by Pfizer. Medical writing assistance for this manuscript was provided by Lisa Shannon, PharmD, and Katie Gersh, Ph.D. of MedErgy, and was funded by Pfizer.
Conflicts of interest
J.-M. Kaufman reports consulting fees, paid advisory boards, lecture fees, and/or grant support from Amgen, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche, Sanofi-Aventis, Servier, and Warner Chilcott as well as investigator fees from Wyeth (presently Pfizer) for the clinical trial described in the present manuscript. S. Palacios has been a symposium speaker or advisory board member for Servier, Pfizer, Pierre-Fabre, Bayer Schering Pharma, GlaxoSmithKline, and Amgen and has received research grants and/or consulting fees from Pfizer, Servier, Amgen, Bayer Schering Pharma, and Teva. S. Sutradhar is a current employee of Pfizer Inc. A.A. Chines is a stockholder and former employee of Pfizer. S. Silverman has no relevant disclosures to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Chines is no longer affiliated with Pfizer.
Rights and permissions
About this article
Cite this article
Kaufman, JM., Palacios, S., Silverman, S. et al. An evaluation of the Fracture Risk Assessment Tool (FRAX®) as an indicator of treatment efficacy: the effects of bazedoxifene and raloxifene on vertebral, nonvertebral, and all clinical fractures as a function of baseline fracture risk assessed by FRAX® . Osteoporos Int 24, 2561–2569 (2013). https://doi.org/10.1007/s00198-013-2341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2341-6