Abstract
Summary
It is important to establish the relationship between pentosidine and advanced glycation endproducts (AGEs) in bone. We found the relationship between pentosidine and AGEs and their magnitude of accumulation were dependent on bone’s surface-to-volume ratio. Results illustrate the importance of measuring pentosidine and AGEs separately in cancellous and cortical bone.
Introduction
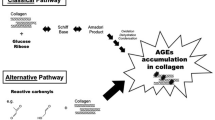
Accumulation of collagen cross-links (AGEs) produced by non-enzymatic glycation deteriorates bone’s mechanical properties and fracture resistance. Although a single AGE, pentosidine, is commonly used as a representative marker, it is unclear whether it quantitatively reflects total fluorescent AGEs in bone. The goal of this study was to establish the relationship between pentosidine and total AGEs in cancellous and cortical bone.
Methods
Pentosidine and total AGEs were quantified in 170 human bone samples. Total fluorescent AGEs were measured in 28 additional cancellous and cortical bone specimens of the same apparent volume that were incubated in control or in vitro glycation solutions. Correlations between pentosidine and total AGEs and differences between cortical and cancellous groups were determined.
Results
Pentosidine was correlated with total AGEs in cancellous bone (r = 0.53, p < 0.0001) and weakly correlated in cortical bone (r = 0.23, p < 0.05). There was more pentosidine (p < 0.01) and total AGEs (p < 0.001) in cancellous than in cortical bone. The in vitro glycation substudy showed that cancellous bone accumulated more AGEs than cortical bone (p < 0.05).
Conclusion
The relationship between pentosidine and total AGEs and their magnitude of accumulation differed in cancellous and cortical bone of the same apparent volume, and were dependent on the surface-to-volume ratios of each sample. It is important to consider the bone types as two separate entities, and it is crucial to quantify total AGEs in addition to pentosidine to allow for more comprehensive analysis of the effects of non-enzymatic glycation in bone.




Similar content being viewed by others
References
Paul RG, Bailey AJ (1996) Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol 28:1297–1310
Bailey AJ, Paul RG, Knott L (1998) Mechanisms of maturation and ageing of collagen. Mech Ageing Dev 106:1–56
Vashishth D (2007) The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep 5:62–66
Robins SP, Bailey AJ (1972) Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem Biophys Res Commun 48:76–84
Knott L, Bailey AJ (1998) Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone 22:181–187
Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ (2002) Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J 364:1–14
Tang SY, Zeenath U, Vashishth D (2007) Effects of non-enzymatic glycation on cancellous bone fragility. Bone 40:1144–1151
Saito M, Marumo K, Fujii K, Ishioka N (1997) Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem 253:26–32
Sroga GE, Vashishth D (2011) UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen. J Chromatogr B Analyt Technol Biomed Life Sci 879:379–385
Vashishth D (2009) Advanced glycation end-products and bone fractures. IBMS BoneKEy 6:268–278
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7
Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM (2005) Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37:825–832
Dyer DG, Blackledge JA, Thorpe SR, Baynes JW (1991) Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem 266:11654–11660
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17:319–336
Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP (2001) Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28:195–201
Gross J (1958) Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. J Exp Med 107:247–263
Norman TL, Yeni YN, Brown CU, Wang Z (1998) Influence of microdamage on fracture toughness of the human femur and tibia. Bone 23:303–306
Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A (2005) Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci 1043:710–717
Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML (2006) Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone 39:1073–1079
Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D (2009) Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 20:887–894
Ding M, Hvid I (2000) Quantification of age-related changes in the structure model type and trabecular thickness of human tibial cancellous bone. Bone 26:291–295
Müller R, Gerber SC, Hayes WC (1998) Micro-compression: a novel technique for the nondestructive assessment of local bone failure. Technol Health Care 6:433–444
Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P (1999) Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167–1174
Karim L, Vashishth D (2012) Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One 7:e35047
Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P (2007) Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem 282:5691–5703
Hildebrand T, Rüegsegger P (1997) Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Eng 1:15–23
Mundy GR (2000) Pathogenesis of osteoporosis and challenges for drug delivery. Adv Drug Deliv Rev 42:165–173
Acknowledgments
This study was funded by National Institute on Aging grant AG20618 (Vashishth) and National Institute of General Medical Sciences training grant T32 GM067545 (Karim). Human cadaver bones were obtained from the International Institute for the Advancement of Medicine and also from the National Disease Research Interchange through the National Institutes of Health grant 5 U42 RR006042.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karim, L., Tang, S.Y., Sroga, G.E. et al. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int 24, 2441–2447 (2013). https://doi.org/10.1007/s00198-013-2319-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2319-4