Abstract
Summary
This study evaluates the effect of hydrolyzed collagen (HC) on bone health of ovariectomized mice (OVX) at different ages. Twenty-six weeks after the OVX procedure, HC ingestion was still able to improve significantly bone mineral density (BMD) and some femur biomechanical parameters. Moreover, HC ingestion for 1 month before surgery prevented BMD decrease.
Introduction
HC can play an important role in preserving BMD before osteoporosis appears. The aim of this study was to evaluate the effect of HC on bone health of ovariectomized mice at different ages.
Methods
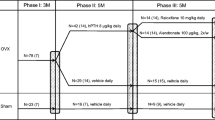
Female C3H mice were either OVX at 3 or 6 months and fed for 6 months (first experiment) or 3 months (second experiment) with diet including 0, 10, or 25 g/kg of HC. In the second experiment, one group received HC 1 month before surgery, and two groups received the supplementation immediately after surgery, one fed ad libitum and the other by gavage. Mice treated with raloxifene were used as a positive control. BMD, femur intrinsic and extrinsic biomechanical properties, and type I collagen C-terminal telopeptide were measured after 12 and 26 weeks. Food intake and spontaneous physical activity were also recorded.
Results
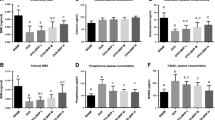
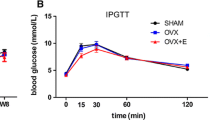
The OVX procedure increased body weight, while food intake decreased, thus suggesting that resting metabolism was decreased. Ingestion of 25 g/kg of HC for 3 or 6 months reduced bone loss significantly in, respectively, 3- and 6-month-old OVX mice. The lowest HC concentration was less efficient. HC ingestion for 3 months is as efficient as raloxifene to protect 3-month-old OVX mice from bone loss. Our results also demonstrated that HC ingestion before surgery prevented the BMD decreases.
Conclusion
This study confirms that dietary collagen reduces bone loss in OVX mice by increasing the diameter of the cortical areas of femurs and can have a preventive effect.



Similar content being viewed by others
References
Omi N, Ezawa I (1995) The effect of ovariectomy on bone metabolism in rats. Bone 17:163S–168S
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14:S13–S18
Burr DB (2002) The contribution of the organic matrix to bone's material properties. Bone 3:8–11
Currey JD (2001) Bone strength: what are we trying to measure? Calcif Tissue Int 68:205–210
Seeman E, Delmas PD (2006) Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261
Turner CH (2006) Bone strength: current concepts. Ann N Y Acad Sci 1068:429–446
Peters BS, Martini LA (2010) Nutritional aspects of the prevention and treatment of osteoporosis. Arq Bras Endocrinol Metabol 54:179–185
Bonjour JP (2005) Dietary protein: an essential nutrient for bone health. J Am Coll Nutr 24:526S–536S
Kerstetter JE, O'Brien KO, Insogna KL (1998) Dietary protein affects intestinal calcium absorption. Am J Clin Nutr 68:859–865
Lips P, Bouillon R, van Schoor NM, Vanderschueren D, Verschueren S, Kuchuk N, Milisen K, Boonen S (2010) Reducing fracture risk with calcium and vitamin D. Clin Endocrinol 73:277–285
Thissen JP, Ketelslegers JM, Underwood LE (1994) Nutritional regulation of the insulin-like growth factors. Endocr Rev 15:80–101
Schürch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP (1998) Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 128:801–809
Barzel US (1995) The skeleton as an ion exchange system: implications for the role of acid-base imbalance in the genesis of osteoporosis. J Bone Miner Res 10:1431–1436
Barzel US, Massey LK (1998) Excess dietary protein can adversely affect bone. J Nutr 128:1051–1053
Moskowitz RW (2000) Role of collagen hydrolysate in bone and joint disease. Semin Arthritis Rheum 30:87–99
Koyama Y, Hirota A, Mori H, Takahara H, Kuwaba K, Kusubata M, Matsubara Y, Kasugai S, Itoh M, Irie S (2001) Ingestion of gelatin has differential effect on bone mineral density and body weight in protein undernutrition. J Nutr Sci Vitaminol 47:84–86
Wu J, Fujioka M, Sugimoto K, Mu G, Ishimi Y (2004) Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J Bone Miner Metab 22:547–553
Nomura Y, Oohashi K, Watanabe M, Kasugai S (2005) Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition 21:1120–1126
Guillerminet F, Beaupied H, Fabien-Soulé V, Tomé D, Benhamou CL, Roux C, Blais A (2010) Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: an in vitro and in vivo study. Bone 46:827–834
Ohara H, Matsumoto H, Ito K, Iwai K, Sato K (2007) Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem 55:1532–1535
Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ (2007) Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 28:3228–3235
Nagy TR, Clair AL (2000) Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8:392–398
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608
Di Masso RJ, Font MT, Capozza RF, Detarsio G, Sosa F, Ferretti JL (1997) Long-bone biomechanics in mice selected for body conformation. Bone 20:539–545
Blais A, Malet A, Mikogami T, Martin-Rouas C, Tome D (2009) Oral bovine lactoferrin improves bone status of ovariectomized mice. Am J Physiol Endocrinol Metab 296:E1281–E1288
Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG (2005) Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 20:1085–1092
Malet A, Bournaud E, Lan A, Mikogami T, Tome D, Blais A (2011) Bovine lactoferrin improves bone status of ovariectomized mice via immune function. Bone 48:1028–1035
Jochems C, Lagerquist M, Håkansson C, Ohlsson C, Carlsten H (2008) Long-term anti-arthritic and anti-osteoporotic effects of raloxifene in established experimental postmenopausal polyarthritis. Clin Exp Immunol 152:593–597
Schroeder TM, Jensen ED, Westendorf JJ (2005) Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 75:213–225
Cano A, Dapía S, Noguera I, Pineda B, Hermenegildo C, Del Val R, Caeiro JR, García-Pérez MA (2008) Comparative effects of 17β-estradiol, raloxifene and genistein on bone 3D microarchitecture and volumetric bone mineral density in the ovariectomized mice. Osteoporos Int 19:793–800
Sliwiński L, Folwarczna J, Nowińska B, Cegieła U, Pytlik M, Kaczmarczyk-Sedlak I, Trzeciak H, Trzeciak HI (2009) A comparative study of the effects of genistein, estradiol and raloxifene on the murine skeletal system. Acta Biochim Pol 2:261–270
Marie PJ (2008) Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys 473:98–105
Burr DB (2002) The contribution of the organic matrix to bone's material properties. Bone 31(1):8–11
Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, Aspden RM, Ralston SH (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107(7):899–907
Li CY, Schaffler MB, Wolde-Semait HT, Hernandez CJ, Jepsen KJ (2005) Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res 20:2150–2158
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This document contains proprietary data and other information within the meaning of article 21 of EU Regulation (EC) no. 1924/2006 (the “Regulation”). No parties other than the authors and their respective employers have a right to use or refer to this data and information within the meaning of this Regulation.
Rights and permissions
About this article
Cite this article
Guillerminet, F., Fabien-Soulé, V., Even, P.C. et al. Hydrolyzed collagen improves bone status and prevents bone loss in ovariectomized C3H/HeN mice. Osteoporos Int 23, 1909–1919 (2012). https://doi.org/10.1007/s00198-011-1788-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1788-6