Abstract
Summary
The vertebral endplates in lumbar radiographs were located by a semi-automatic annotation method using statistical shape models.
Introduction
Vertebral fractures are common osteoporotic fractures, but current quantitative detection methods (morphometry) lack specificity. We have previously developed more specific quantitative classifiers of vertebral fracture using shape and appearance models. This method has only been applied to DXA vertebral fracture assessment (VFA) images and not to spinal radiographs. The classifiers require a detailed annotation of the outline of the vertebral endplate, so we investigated the application of similar semi-automated annotation methods to lumbar radiographs as the initial step in the generalisation of the statistical classifiers used in VFA images.
Methods
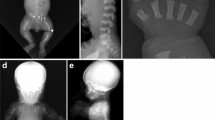
The vertebral body outlines in a training set of 670 lumbar radiographs were manually annotated by expert radiologists. This training set was used to build statistical models of vertebral shape and appearance using triplets of vertebrae. In order to segment vertebrae, the models were refitted using a sequence of active appearance models of vertebral triplets, using a miss-40-out train/test cross-validation experiment. The accuracy was evaluated against the manual annotation and analysed by fracture grade.
Results
Good accuracy was obtained for normal vertebrae (0.82 mm) and fracture grades 1 and 2 (1.19 mm), but the localisation accuracy deteriorated for grade 3 fractures to 2.12 mm.
Conclusion
Vertebral body shape annotation can be substantially automated on lumbar radiographs. However, an occasional manual correction may be required, typically with either severe fractures, or when there is a high degree of projectional tilting or scoliosis. The located detailed shapes may enable the development of more powerful quantitative classifiers of osteoporotic vertebral fracture.




Similar content being viewed by others
References
Ensrud K, Thompson D, Cauley J, Nevitt M, Kado D, Hochberg M, An S, Black D (2000) Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 48:241–249
Melton LJ III, Atkinson EJ, Cooper C et al (1999) Vertebral fractures predict subsequent fractures. Osteoporos Int 10(3):214–221
Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285(3):320–323
Ensrud KE, Nevitt MC, Palermo L et al (1999) What proportion of incident morphometric vertebral fractures are clinically diagnosed and vice versa? J Bone Miner Res 14(S1):S138
Gehlbach SH, Bigelow C, Heimisdottir M et al (2000) Recognition of vertebral fractures in a clinical setting. Osteoporos Int 11(7):577–582
Delmas PD, van de Langerijt L, Watts NB et al (2005) Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 20(4):557–563
Ferrar L, Jiang G, Adams J, Eastell R (2005) Identification of vertebral fractures: an update. Osteoporos Int 16(7):717–728
Guermazi A, Mohr A, Grigorian M et al (2002) Identification of vertebral fractures in osteoporosis. Semin Musculoskelet Radiol 6(3):241–252
Genant HK, Wu CY, van Kuijk C et al (1993) Vertebral fracture assessment using a semi-quantitative technique. J Bone Miner Res 8(9):1137–1148
Black DM, Cummings SR, Stone K et al (1991) A new approach to defining normal vertebral dimensions. J Bone Miner Res 6(8):883–892
Eastell R, Cedel SL, Wahner HW et al (1991) Classification of vertebral fractures. J Bone Miner Res 6(3):207–215
McCloskey E, Spector T, Eyres K et al (1993) The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int 3(3):138–147
Guglielmi G, Diacinti D, van Kuijk C et al (2008) Vertebral morphometry: current methods and recent advances. Eur Radiol 18(7):1484–1496
Wu CY, Li J, Jergas M et al (1995) Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int 5(5):354–370
Black D, Palermo L, Nevitt MC et al (1995) Comparison of methods for defining prevalent vertebral deformities: the study of osteoporotic fractures. J Bone Miner Res 10(6):890–902
Jiang G, Eastell R, Barrington NA, Ferrar L (2004) Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 15(11):887–896
Roberts MG, Pacheco EM, Mohamkumar R, Cootes TF, Adams JE (2010) Detection of vertebral fractures in DXA VFA images using statistical models of appearance and a semi-automatic segmentation. Osteoporos Int 21:2037–2046
de Bruijne M, Lund M, Tanko L et al (2007) Quantitative vertebral morphometry using neighbour-conditional shape models. Med Image Anal 11(5):503–512
Cootes TF, Edwards GJ, Taylor CJ (1998) Active appearance models. In: Burkhardt H, Neumann B (eds) Proc. 5th European conference on computer vision. Springer, Berlin, pp 484–498
Cootes TF, Edwards GJ, Taylor CJ (2001) Active appearance models. IEEE Trans Pattern Match Mach Intell 23(6):681–885
Roberts MG, Cootes TF, Adams JE (2006) Vertebral morphometry: semiautomatic determination of detailed shape from dual-energy x-ray absorptiometry images using active appearance models. Invest Radiol 41(12):849–859
Cooper C, Shah S, Hand DJ et al (1991) Screening for vertebral osteoporosis using individual risk factors. Osteoporos Int 2:48–53
Gluer C, Eastell R, Reid DM et al (2004) Association of five quantitative ultrasound devices and bone densitometry with osteoporotic vertebral fractures in a population-based sample: the OPUS study. J Bone Miner Res 19:782–793
Ferrar L, Jiang G, Barrington NA, Eastell R (2000) Identification of vertebral deformities in women: comparison of radiological assessment and quantitative morphometry using morphometric radiography and morphometric x-ray absorptiometry. J Bone Miner Res 15(3):575–585
Scott IM, Cootes TF, Taylor CJ (2003) Improving active appearance model matching using local image structure. In: Proceedings of the 18th conference on information processing in medical imaging, Ambleside, UK, July 20–25, 2003, pp 258–269
Roberts MG (2008) Automatic detection and classification of vertebral fracture using statistical models of appearance. PhD thesis, University of Manchester
Howe B, Gururajan A, Sari-Sarraf H et al. (2004) Hierarchical segmentation of cervical and lumbar vertebrae using a customized generalized hough transform and extensions to active appearance models. In: Proceedings IEEE 6th SSIAI, Lake Tahoe, NV, March 2004, 182–186
de Bruijne M, Nielsen M. (2004) Image segmentation by shape particle filtering. In: Proc. International Conference on Pattern Recognition 2004, 722–725
Ettinger B, Black DM (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3 year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) investigators. JAMA 282(7):637–645
Siris E, Genant HK, Laster AJ et al (2007) Enhanced prediction of fracture risk combining vertebral fracture and BMD. Osteoporos Int 18(6):761–770
Neer RM, Arnaud CD, Zanetta JR et al (2001) Effect of parathyroid hormone (1–34) on bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441
Genant HK, Delmas PD, Chen P et al (2007) Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int 18(1):69–76
Parfitt AM, Mathews CH (1983) Relations between surface, volume and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest 72:1396–1409
Roberts MG, Cootes TF, Pacheco E, Adams JE (2007) Quantitative vertebral fracture detection on DXA images using shape and appearance models. Acad Radiol 14(10):1166–1178
Acknowledgements
The authors acknowledge the kind provision of digitised training images by the team at the University of Sheffield (Professor R. Eastell and Dr. L. Ferrar). We thank Professor Cyrus Cooper (Universities of Southampton and Oxford) and Professor David Reid (University of Aberdeen) for permission to use radiographs from earlier studies. The authors wish to thank Mr Stephen Capener (SC) who performed the manual annotation of the vertebrae in the first dataset. The work was funded through a grant from Arthritis Research UK, with earlier foundation work having been funded by grants from the Central Manchester University Hospitals NHS Foundation Trust (CMFT) Research Endowment Fund.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix 1—training set annotation tool
Appendix 1—training set annotation tool
The images used for training were annotated manually using an in-house tool. The tool partly used previous AAMs to reduce the manual labour, with the models being iteratively updated as the annotated cases were added to the dataset. The current AAM set can be used to approximately locate the vertebrae. Then, the user moves any points which are not adequately located. A useful feature of this tool is that when points have been manually repositioned, they constrain other points in the model; so, if the initial automatic fit is incorrect, the user can partially correct it and then re-run the model fit as constrained by the corrections. For example, when four corner points and four mid-points (inner and outer superior and inferior rims) of a vertebra have been manually positioned the partially trained current AAM will typically produce good convergence on the rest of the vertebral shape in most cases, although fractured vertebrae may well need a few more points to be manually positioned due to their more complex shape.
There can be vertebrae (e.g. severe fractures) to which the provisional shape model cannot be fitted, due to undertraining. Therefore, we also used a dynamic programming edge search method in the tool. This first fits the vertebra's shape model learned so far to points already positioned by the user (e.g. the 4 corner points). Typically, this will not fit precisely due to undertraining, so next, the whole shape is warped using thin plate splines so that the shape exactly passes through the user-determined points. Then, those points not fixed by the user are moved towards the strongest local edge within 3 mm, but with high curvature penalised, and the optimum shape for the best combination of edge and curvature penalty costs is determined by dynamic programming (DP). If necessary, the user can further constrain the solution by fixing more points, or the user can accept the warped model fit prior to the DP edge–fit stage (e.g. in regions where there is no strong edge).
Hence, the tool is not constrained by the prototype models in cases where there is an inadequate fit; but as the annotated set is built up, it is possible to use the existing shape and appearance statistics to speed up annotating further vertebrae. Each time a new batch of images was annotated, all models were re-built. In the early stages, prototype shape models were created based on existing DXA VFA models, with a priori estimates of projective tilting effects using Bezier splines for the additional endplate rims. In the earlier batches of images (e.g. first 100 images), the AAMs were not used, and instead, the DP edge–fit method was used, and it was necessary to manually position a much higher proportion of points than in the later stages in which the models were becoming better trained.
Rights and permissions
About this article
Cite this article
Roberts, M.G., Oh, T., Pacheco, E.M.B. et al. Semi-automatic determination of detailed vertebral shape from lumbar radiographs using active appearance models. Osteoporos Int 23, 655–664 (2012). https://doi.org/10.1007/s00198-011-1604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1604-3