Abstract
Summary
Postmenopausal women with osteoporosis received 75 mg risedronate on two consecutive days each month or 5 mg daily for 12 months. Changes in bone mineral density and bone turnover markers were similar between treatments. Risedronate 75 mg twice monthly was effective and safe suggesting a new, convenient dosing schedule.
Introduction
Patients perceive less frequent dosing as being more convenient. This 2-year trial evaluates the efficacy and safety of a new monthly oral regimen of risedronate; 1 year results are presented here.
Methods
Postmenopausal women with osteoporosis (n = 1229) were randomly assigned to double-blind treatment with 75 mg risedronate on two consecutive days each month (2CDM), or 5 mg daily. The primary endpoint was the percent change from baseline in lumbar spine (LS) bone mineral density (BMD) at month 12. Secondary efficacy was evaluated by mean percent changes from baseline in BMD in LS, total hip, trochanter, and femoral neck, and bone turnover markers (BTMs).
Results
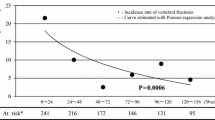
Risedronate 75 mg 2CDM was non-inferior to 5 mg daily (treatment difference 0.21; 95% CI -0.19 to 0.62). Mean percent change in LS-BMD was 3.4% ± 0.16 and 3.6% ± 0.15 respectively. Mean percent changes in BMD and BTMs were significant and similar for both treatment groups. New vertebral fractures occurred in 1% of subjects with either treatment. Both treatments were generally well tolerated and safe.
Conclusions
Risedronate 75 mg 2CDM was non-inferior in efficacy and did not show a difference in safety vs. 5 mg daily after 12 months, leading to a similar benefit.



Similar content being viewed by others
References
Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38(2 suppl 1):S4–S9
North American Menopause Society (2006) Management of osteoporosis in postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause 13:340–367
Brown JP, Kendler DL, McClung MR et al (2002) The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 71:103–111
Emkey R (2004) Alendronate and risedronate for the treatment of postmenopausal osteoporosis: clinical profiles of the once-weekly and once-daily dosing formulations. MedGenMed 6:6
Delmas PD, Recker RR, Chesnut CH III et al (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15:792–798
Miller PD, McClung MR, Macovei L et al (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 20:1315–1322
Keen R, Jodar E, Iolascon G et al (2006) European women’s preference for osteoporosis treatment: influence of clinical effectiveness and dosing frequency. Curr Med Res Opin 22:2375–2381
Gold DT, Safi W, Trinh H (2006) Patient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate. Curr Med Res Opin 22:2383–2391
Weiss TW, Gold DT, Silverman SL et al (2006) An evaluation of patient preferences for osteoporosis medication attributes: results from the PREFER-US study. Curr Med Res Opin 22:949–960
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Reginster J, Minne HW, Sorensen OH et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Sorensen OH, Crawford GM, Mulder H et al (2003) Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone 32:120–126
Harris ST, Watts NB, Li Z et al (2004) Two-year efficacy and tolerability of risedronate once a week for the treatment of women with postmenopausal osteoporosis. Curr Med Res Opin 20:757–764
McClung MR, Geusens T, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
McClung MR, Purple CR, Zhou X et al (2007) Risedronate at both 2.5 mg and 5.0 mg daily doses reduces the risk of hip fracture in women with established osteoporosis. Osteoporos Int 18(suppl 2):S215
Delmas PD, McClung MR, Zanchetta JR et al (in press) Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Bone
Thompson GA, Russell DA, Schnell DJ et al (2006) Risedronate pharmacokinetics following daily and monthly dosing regimes. Calcif Tissue Int 78(Suppl 1):S162
Acknowledgements
The following investigators participated in this study: Argentina: H. Salerni, L. Plantalech, C. Mautalen, Z. Man, J. Zanchetta; Australia: G. Nicholson, T. Diamond, P.S. Brook, S. Hall, P. Nach, J. Karash; Canada: L.G. SteMarie, J. Brown, D. Hanley, A. Hodsman, W. Olszynski, R. Josse, A. Kahn, J. Wade; Czech Republic: P. Kasalichy, V. Palicka, V. Kuba, V. Vyskocil; France: P.D. Delmas, G. Werhya, P. Fardellone, M. Laroche, C.L. Benhamou, M.C. DeVernejoul; United Kingdom: I. Caldwell, T. Maxwell, D. Dutchman; Poland: W. Tlustochowicz, J. Padzur, M. Korkosz, A. Fillopowivz-Sosnowska; Turkey: P. Yalcin, N. Parker, B. Gulekli, C. Yilmaz, N. Dursun; Lebanon: G. Maalouf; United States: M. McClung, M. Greenwald, R. Emkey, W. Ellison, S. Cohen, R. Weinstein, D. Fiske, R. Recker, R. Ackerman, H. Greisberg, J. Funk, T. Hennessey; South Africa: T.J. deVilliers, S. Lipschitz, G.C. Ellis, M. Davey, A.J. de Weerd, S. Hough.
Funding
Funding for this study was provided by the Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals, Cincinnati, OH, USA, and sanofi-aventis, Paris, France). The authors received editorial/writing support in the preparation of this manuscript, funded by the Alliance for Better Bone Health. The authors were fully responsible for contents and editorial decisions for this manuscript.
Conflict of interest statement
Prof. P.D. Delmas has received consultant/honorarium fees and a research grant from Procter and Gamble/sanofi-aventis. Dr Benhamou has received advisory fees and for research grants from Amgen, Lilly, Merck, Novartis, Procter and Gamble, Roche, and Servier. Prof. Dr. Man has received consulting fees or advisory fees from Novartis and lectures fees from Aventis, sanofi-aventis and Roche. Dr. Tlustochowicz has received a research grant from Procter and Gamble/sanofi-aventis. Dr Matzkin is an employee of sanofi-aventis. Dr Eusebio is an employee of Procter and Gamble. Dr. Zanchetta has received consultant fees and/or research grants from Sanofi-Aventis, GlaxoSmithKline, Merck, Lilly, Wyeth, Amgen, Pfizer, Roche and Servier. Dr. Olszynski has received a research grant from Procter and Gamble/sanofi-aventis. Dr. Recker is a consultant for Merck, Lilly, Procter and Gamble, Wyeth, Amgen, Novartis, and Roche. Dr McClung has received consulting fees and research grants from Amgen, Lilly, Merck, Novartis, Procter and Gamble, Roche and sanofi-aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delmas, P.D., Benhamou, C.L., Man, Z. et al. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int 19, 1039–1045 (2008). https://doi.org/10.1007/s00198-007-0531-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0531-9