Abstract
Introduction
To test the hypothesis that the bone metabolism of a growth-restricted foetus is regulated by genetic, placental and/or foetal factors through leptin, we investigated the foetal bone turnover in monochorionic pregnancies complicated with or without twin–twin transfusion syndrome (TTTS).
Methods
Maternal and cord bloods were collected from gestational-age-matched monochorionic twins with (n = 15) and without (n = 15) TTTS. The samples were assayed for leptin, cross-linked carboxyl terminal telo-peptide (ICTP, a marker of bone resorption) and pro-peptide (PICP, a marker of bone formation) of type I collagen by radioimmunoassay (RIA).
Results
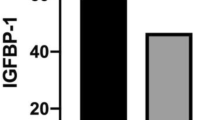
In the growth-restricted donor twin, the plasma concentration of leptin (P < 0.001), PICP (P < 0.001) was lower, while that of ICTP (P < 0.001) was higher than the recipient twin of the TTTS group. In contrast, leptin, PICP and ICTP were comparable in non-TTTS twins. In the recipient twin of TTTS and non-TTTS twins, leptin was positively associated with PICP (r = 0.73; n = 45, P < 0.001) and negatively with ICTP (r = −0.68; n = 45; P < 0.001). No such association was found between leptin and bone marker in the growth-restricted donor twin of the TTTS group.
Conclusion
Our data suggest that, in AGA twins, leptin maintains bone metabolism by inhibiting resorption and enhancing bone formation. In contrast, growth-restricted donor twins have high bone turnover and this does not seem to be due to leptin deficiency.




Similar content being viewed by others
References
Javaid MK, Cooper C (2002) Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab 16(2):349–367
Yarbrough DE, Barrett-Connor E, Morton DJ (2000) Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bernardo Study. Osteoporos Int 11(7):626–630
Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C (2001) Neonatal bone mass: Influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res 16(9):1694–1703
Javaid MK, Godfrey KM, Taylor P, Robinson SM, Crozier SR, Dennison EM, Robinson JS, Breier BR, Arden NK, Cooper C (2005) Umbilical cord leptin predicts neonatal bone mass. Calcif Tissue Int 76(5):341–347
Eckert JE, Gatford KL, Luxford BG, Campbell RG, Owens PC (2000) Leptin expression in offspring is programmed by nutrition in pregnancy. J Endocrinol 165(3):R1–R6
Bajoria R, Kingdom J (1997) The case for routine determination of chorionicity and zygosity in multiple pregnancy. Prenat Diagn 17(13):1207–1225
Bajoria R, Hancock M, Ward S, D’Souza SW, Sooranna SR (2000) Discordant amino acid profiles in monochorionic twins with twin–twin transfusion syndrome. Pediatr Res 48(6):821–828
Risteli L, Risteli J (1993) Biochemical markers of bone metabolism. Ann Med 25(4):385–393
Sooranna SR, Ward S, Bajoria R (2001) Discordant fetal leptin levels in monochorionic twins with chronic midtrimester twin–twin transfusion syndrome. Placenta 22(5):392–398
Petersen S, Gotfredsen A, Knudsen FU (1989) Total body bone mineral in light-for-gestational-age infants and appropriate-for-gestational-age infants. Acta Paediatr Scand 78(3):347–350
Namgung R, Tsang RC, Specker BL, Sierra RI, Ho ML (1993) Reduced serum osteocalcin and 1,25-dihydroxyvitamin D concentrations and low bone mineral content in small for gestational age infants: evidence of decreased bone formation rates. J Pediatr 122(2):269–275
Namgung R, Tsang RC, Sierra RI, Ho ML (1996) Normal serum indices of bone collagen biosynthesis and degradation in small for gestational age infants. J Pediatr Gastroenterol Nutr 23(3):224–228
Kajantie E, Hytinantti T, Koistinen R, Risteli J, Rutanen EM, Seppala M, Andersson S (2001) Markers of type I and type III collagen turnover, insulin-like growth factors, and their binding proteins in cord plasma of small premature infants: relationships with fetal growth, gestational age, preeclampsia, and antenatal glucocorticoid treatment. Pediatr Res 49(4):481–489
Ogueh O, Khastgir G, Studd J, Jones J, Alaghband-Zadeh J, Johnson MR (1998) Maternal and fetal plasma levels of markers of bone metabolism in gestational diabetic pregnancies. Early Hum Dev 53(2):155–161
Demarini S, Specker BL, Sierra RI, Miodovnik M, Tsang RC (1995) Evidence of increased intrauterine bone resorption in term infants of mothers with insulin-dependent diabetes. J Pediatr 126(5 Pt 1):796–798
Nakano K, Iwamatsu T, Wang CM, Tarasima M, Nakayama T, Sasaki K, Tachikawa E, Noda N, Mizoguchi E, Osawa M (2006) High bone turnover of type I collagen depends on fetal growth. Bone 38(2):249–256
Seibold-Weiger K, Wollmann HA, Ranke MB, Speer CP (2000) Plasma concentrations of the carboxyterminal propeptide of type I procollagen (PICP) in preterm neonates from birth to term. Pediatr Res 48(1):104–108
Reid IR, Comish J (2004) Direct actions of leptin on bone remodeling. Calcif Tissue Int 74(4):313–316
Steppan CM, Crawford DT, Chidsey-Frink KL, Ke HZ, Swick AG (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regulatory Pept 92(1–3):73–78
Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC (2002) Leptin inhibits osteoclast generation. J Bone Miner Res 17(2):200–209
Ogueh O, Sooranna S, Nicolaides KH, Johnson MR (2000) The relationship between leptin concentration and bone metabolism in the human fetus. J Clin Endocrinol Metab 85(5):1997–1999
Dennison EM, Syddall HE, Fall CH, Javaid MK, Arden NK, Phillips DI, Cooper C (2004) Plasma leptin concentration and change in bone density among elderly men and women: the Hertfordshire Cohort Study. Calcif Tissue Int 74(5):401–406
Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, Klisovic D, Nahhas RW, Landoll JD (1997) Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 82(10):3239–3245
Odabai E, Ozata M, Turan M, Bingol N, Yonem A, Cakir B, Kutlu M, Ozdemir IC (2000) Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol 142(2):170–173
Iwamoto I, Douchi T, Kosha S, Murakami M, Fujino T, Nagata Y (2000) Relationships between serum leptin level and regional bone mineral density, bone metabolic markers in healthy women. Acta Obstet Gynecol Scand 79(12):1060–1064
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100(2):197–207
Morberg CM, Tetens I, Black E, Toubro S, Soerensen TI, Pedersen O, Astrup A (2003) Leptin and bone mineral density: a cross-sectional study in obese and nonobese men. J Clin Endocrinol Metab 88(12):5795–5800
Sato M, Takeda N, Sarui H, Takami R, Takami K, Hayashi M, Sasaki A, Kawachi S, Yoshino K, Yasuda K (2001) Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab 86(11):5273–5276
Bjorbaek C, Kahn BB (2004) Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res 59:305–331
Matijevic R, Ward S, Bajoria R (2002) Non-invasive method of evaluation of trophoblast invasion of spiral arteries in monochorionic twins with discordant birthweight. Placenta 23(1):93–99
Bajoria R, Gibson MJ, Ward S, Sooranna SR, Neilson JP, Westwood M (2001) Placental regulation of insulin-like growth factor axis in monochorionic twins with chronic twin–twin transfusion syndrome. J Clin Endocrinol Metab 86(7):3150–3056
Bajoria R, Sooranna SR, Ward S, Chatterjee R (2006) Elevated IGFBP-1 cause high bone turnover in growth-restricted monochorionic twins with discordant birth weight. Bone 38(6):929–934
Rosen CJ (2004) Insulin-like growth factor I and bone mineral density: experience from animal models and human observational studies. Best Pract Res Clin Endocrinol Metab 18(3):423–435
Tobias JH, Cooper C (2004) PTH/PTHrP activity and the programming of skeletal development in utero. J Bone Miner Res 19(2):177–182
Kovacs CS, Kronenberg HM (1997) Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18(6):832–872
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423(6937):332–336
Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C; Princess Anne Hospital Study Group (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367(9504):36–43
Colak O, Alatas O, Aydogdu S, Uslu S (2002) The effect of smoking on bone metabolism: maternal and cord blood bone marker levels. Clin Biochem 35(3):247–250
Krieger NS, Frick KK, Bushinsky DA (2004) Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 13(4):423–436
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85(3):632–639
Brandao-Burch A, Utting JC, Orriss IR, Arnett TR (2005) Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int 77(3):167–174
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajoria, R., Sooranna, S.R. & Chatterjee, R. Leptin and bone turnover in monochorionic twins complicated by twin–twin transfusion syndrome. Osteoporos Int 18, 193–200 (2007). https://doi.org/10.1007/s00198-006-0236-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0236-5