Abstract
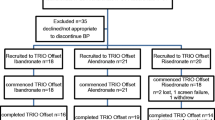
Intravenous pamidronate is frequently used for the treatment of osteoporosis in patients who cannot tolerate oral bisphosphonates. The aim of the present study was to compare the changes in bone mineral density (BMD) after 1 year of treatment with either oral alendronate or intravenous pamidronate in patients with osteoporosis. We studied 40 consecutive patients starting treatment for osteoporosis: 20 received oral alendronate 10 mg/day and 20 received intravenous pamidronate 60 mg/3 months. Patients were started on intravenous pamidronate in the case of intolerance (within 1 month of start of treatment) of an oral bisphosphonate or in the case of contraindications for an oral bisphosphonate. BMD (spine and total hip) was measured with dual X-ray absorptiometry (DEXA) at the start of treatment and after 1 year. The BMD of the lumbar spine increased by 4.0% (P<0.05 vs baseline) in both groups, and the BMD of the hip increased by 3.3% and 2.9% (P<0.05 vs baseline) in the alendronate and pamidronate groups, respectively. The increases in BMD of the vertebral spine and the total hip after 1 year are comparable in the alendronate and pamidronate groups. We conclude that intravenous pamidronate can be used successfully as an alternative treatment in patients with gastrointestinal intolerance of an oral bisphosphonate.
Similar content being viewed by others
References
Riggs BL, Melton LJ III (1992) The prevention and treatment of osteoporosis. N Engl J Med 327:620–627
Widler L, Jaeggi KA, Glatt M, Muller K, Bachmann R, Bisping M, Born AR, Cortesi R, Guiglia G, Jeker H, Klein R, Ramseier U, Schmid J, Schreiber G, Seltenmeyer Y, Green JR (2002) Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa). J Med Chem 45:3721–3738
Fitton A, McTavish D (1991) Pamidronate. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs 41:289–318
Thiebaud D, Burckhardt P, Melchior J, Eckert P, Jacquet AF, Schnyder P, Gobelet C (1994) Two years’ effectiveness of intravenous pamidronate (APD) versus oral fluoride for osteoporosis occurring in the postmenopause. Osteoporos Int 4:76–83
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Peretz A, Body JJ, Dumon JC, Rozenberg S, Hotimski A, Praet JP, Moris M, Ham H, Bergmann P (1996) Cyclical pamidronate infusions in postmenopausal osteoporosis. Maturitas 25:69–75
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Foxamax International Trial Study Group. Osteoporos Int 9:461–468
Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ (1998) Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med 338:485–492
Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, Musliner T, Freedholm D (2000) Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 160:517–525
de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK (1996) Esophagitis associated with the use of alendronate. N Engl J Med 335:1016–1021
Miller NH (1997) Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med 102:43–49
Yood RA, Emani S, Reed JI, Lewis BE, Charpentier M, Lydick E (2003) Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 14:965–968
Ettinger B, Pressman A, Schein J (1998) Clinic visits and hospital admissions for care of acid-related upper gastrointestinal disorders in women using alendronate for osteoporosis. Am J Manag Care 4:1377–1382
Kanis JA, Oden A, Johnell O, Caulin F, Bone H, Alexandre JM, Abadie E, Lekkerkerker F (2002) Uncertain future of trials in osteoporosis. Osteoporos Int 13:443–449
Khosla S (2003) Surrogates for fracture endpoints in clinical trials. J Bone Miner Res 18:1146–1149
Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87:1586–1592
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vis, M., Bultink, I.E.M., Dijkmans, B.A.C. et al. The effect of intravenous pamidronate versus oral alendronate on bone mineral density in patients with osteoporosis. Osteoporos Int 16, 1432–1435 (2005). https://doi.org/10.1007/s00198-005-1862-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1862-z