Abstract
Introduction
It has been suggested that bone mineral density (BMD) measurements should be made at multiple sites, and that the lowest T–score should be taken for the purpose of diagnosing osteoporosis.
Purpose
The aim of this study was to examine the use of BMD measurements at the femoral neck and lumbar spine alone and in combination for fracture prediction.
Methods
We studied 19,071 individuals (68% women) from six prospective population-based cohorts in whom BMD was measured at both sites and fracture outcomes documented over 73,499 patient years. BMD values were converted to Z-scores, and the gradient of risk for any osteoporotic fracture and for hip fracture was examined by using a Poisson model in each cohort and each gender separately. Results of the different studies were merged using weighted β-coefficients.
Results
The gradients of risk for osteoporotic fracture and for hip fracture were similar in men and women. In men and women combined, the risk of any osteoporotic fracture increased by 1.51 [95% confidence interval (CI)=1.42–1.61] per standard deviation (SD) decrease in femoral-neck BMD. For measurements made at the lumbar spine, the gradient of risk was 1.47 (95% CI=1.38–1.56). Where the minimum of the two values was used, the gradient of risk was similar (1.55; 95% CI=1.45–1.64). Higher gradients of risk were observed for hip fracture outcomes: with BMD at the femoral neck, the gradient of risk was 2.45 (95% CI=2.10–2.87), with lumbar BMD was 1.57 (95% CI=1.36–1.82), and with the minimum value of either femoral neck and lumbar spine was 2.11 (95% CI=1.81–2.45). Thus, selecting the lowest value for BMD at either the femoral neck or lumbar spine did not increase the predictive ability of BMD tests. By contrast, the sensitivity increased so that more individuals were identified but at the expense of specificity. Thus, the same effect could be achieved by using a less stringent T–score for the diagnosis of osteoporosis.
Conclusions
Since taking the minimum value of the two measurements does not improve predictive ability, its clinical utility for the diagnosis of osteoporosis is low.
Similar content being viewed by others
References
Anonymous (1993) Consensus development conference: diagnosis, prophylaxis and treatment of osteoporosis. Am J Med 94:646–650
World Health Organisation (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Technical Report Series, vol 843. WHO, Geneve
Kanis JA, Gluer CC, for the Committee of Scientific Advisors, IOF (2000) An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int 11:192–202
Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL et al (1997) Prevalence of low femoral bone density in older US adults from NHANES III. J Bone Miner Res 12:1761–1768
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–486
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J 312:1254–1259
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas PD et al (2005) Predictive value of bone mineral density for hip and other fractures. J Bone Miner Res 20:1185–1194
Lewiecki EM, Kandler DL, Kiebzak GM, Schmeer P, Prince RL, El Hajj Fuleihan G, Hans D (2004) Special report on the official positions of the International Society for Clinical Densitometry. Osteoporos Int 15:779–784
Lewiecki EM, Watts NB, McClung MR et al for the International Society for Clinical Densitometry (2004) Official position of the International Society for Clinical Densitometry. J Clin Endocrinol Metab 89:3651–3655
Kreiger N, Tenenhouse A, Joseph L et al (1999) The Canadian Multicenter Osteoporosis Study (CaMos): background. Rationale. Methods. Canadian J Aging 18:376–387
Jones G, Nguyen TV, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA (1994) Symptomatic fracture incidence in elderly men and women. The Dubbo osteoporosis epidemiology study DOES. Osteoporos Int 4:277–282
Nguyen TV, Eisman JA, Kelly PJ, Sambrook PN (1996) Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol 144:255–263
O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ (1996) The prevalence of vertebral deformity in European men and women: European vertebral osteoporosis study. J Bone Miner Res 11:1010–1017
Felsenberg D, Silman AJ, Lunt M, Ambrecht G, Ismail AA, Finn JD, Cockerill WC, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, Cannata JB, Cooper C, Dequeker J, Eastell R, Ershova O, Felsch B, Gowin W, Havelka S, Hoszowski K, Jajic I, Janot J, Johnell O, Kanis JA, Kragl G, Lopez Vaz A, Lorenc R, Lyritis G, Masaryk P, Matthis C, Miazgowski T, Parisi G, Pols HAP, Poor G, Raspe HH, Reid DM, Reisinger W, Scheidt–Nave C, Stepan J, Todd C, Weber K, Woolf AD, Reeve J, O’Neill TW (2002) Incidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS). J Bone Miner Res 17:716–724
Ismail AA, Pye SR, Cockerill WC, Lunt M, Silman AJ, Reeve J, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, Cannata JB, Cooper C, Delmas PD, Dequeker J, Dilsen G, Falch JA, Felsch B, Felsenberg D, Finn JD, Gennari C, Hoszowski K, Jajic I, Janott J, Johnell O, Kanis JA, Kragl G, Lopez Vaz A, Lorenc R, Lyritis G, Marchand F, Masaryk P, Matthis C, Miazgowski T, Naves–Diaz M, Pols HAP, Poor G, Rapado A, Raspe HH, Reid DM, Reisinger W, Scheidt–Nave C, Stepan J, Todd C, Weber K, Woolf AD, O’Neill TW (2002) Incidence of limb fracture across Europe: results from the European prospective osteoporosis study (EPOS). Osteoporos Int 13:565–571
Fujiwara S, Fumiyoshi K, Masunari N, Naito K, Suzuki G, Fukunage M (2003) Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res 18:1547–1553
Fujiwara S, Kasagi F, Yamada M, Kodama K (1997) Risk factors for hip fracture in Japanese cohort. J Bone Miner Res 12:998–1004
Honkanen R, Tuppurainen M, Kroger H, Alhava E, Saarikoski S (1998) Relationship between risk factors and fracture differ by type of fracture: a population-based study of 12,191 perimenopausal women. Osteoporos Int 8:25–31
Kroger H, Huopio J, Honkanen R, Tupparainen M, Puntila E, Alhava E, Saarikoski S (1995) Prediction of fracture risk using axial bone mineral density in a perimenopausal population: a prospective study. J Bone Miner Res 10:302–306
Melton LJ III, Crowson CS, O’Fallon WM, Wahner HW, Riggs BL (2003) Relative contributions of bone density. bone turnover and clinical risk factors to long-term fracture prediction. J Bone Miner Res 18:312–318
Melton LJ III, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL (1998) Bone density and fracture risk in men. J Bone Miner Res 13:1915–1923
Kanis JA, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12:417–427
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327:557–560
O´Neill TW, Lunt M, Felsenberg D, Benevolenskaya LI, Bhalla AK, Cannata JB, Cooper C, Crabtree N, Dequeker J, Hoszowski K, Jajic I, Kanis JA, Kragl G, Lopes Vaz A, Lorenc R, Lyritis G, Masaryk P, Miazgowski T, Parisi G, Pols HAP, Poor G, Reid DM, Scheidt–Nave C, Stepan J, Todd C, Weber K, Woolf AD, Reeve J (2002) The relationship between bone density and incident vertebral fracture in men and women. J Bone Miner Res 17:2214–2221
DeLaet CEDH, Van Hout BA, Burger H, Hofman A, Weel AEAM, Pols HAP (1998) Hip fracture prediction in elderly men and women: validation in the Rotterdam Study. J Bone Miner Res 13:1587–1593
Cauley JA, Zmuda JM, Wisniewski SR, Krishnaswami S, Palermo L, Stone KL, Black DM, Nevitt MC (2004) Bone mineral density and prevalent vertebral fractures in men and women. Osteoporos Int 15:32–37
Wasnich RD, Davis JW, Ross PD (1994) Spine fracture risk is predicted by non spine fractures. Osteoporos Int 4:1–5
Kanis JA, Johnell O, Oden A, De Laet C, Mellstrom D (2001) Diagnosis of osteoporosis and fracture threshold in men. Calcified Tissue Int 69:218–221
Lunt M, Felsenberg D, Reeve J, Benevolenskaya L, Cannata J, Dequeker J (1997b) Bone density variation and its effects on risk of vertebral deformity in men and women studied in 13 European centres: the EVOS study. J Bone Miner Res 12:1883–1894
Blake GM, Patel R, Knapp KM, Fogelman I (2003) Does the combination of two BMD measurements improve fracture discrimination. J Bone Miner Res 18:1955–1963
Kanis JA, Barton I, Johnell O (2004) Risedronate decreases fracture risk in patients selected solely on the basis of prior vertebral fracture. Osteoporos Int 16:475–482
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Acknowledgements
We are grateful to the Alliance for Better Bone Health, Hologic, IGEA, Lilly, Lunar, Novartis, Pfizer, Roche, Servier, Wyeth, the EU (FP3/5; QLK6–CT–2002–00491) for supporting this study and the International Osteoporosis Foundation, the International Society for Clinical Densitometry and the National Osteoporosis Foundation for their unrestricted support of this work.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
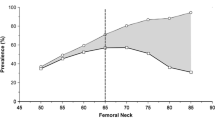
The proportion of individuals with at least one type of BMD measurements below a cut off
Let Ai denote the event that the measurement of type i is below a cut off g. We use the symbols ∪ and ∩ for the union and the intersection of events, respectively. The probability that any of n measurements is below the cut off is
The following notations are used
Then the relationship below holds
The argument (−1)i+1 obviously can only take the values +1 or −1. This is because the first argument of the summation (S1) double counts some combination areas of A1 A2 A3 etc. Those probabilities have to be subtracted in the second argument, but since too much is subtracted, there needs to be some adding in the third argument and so on…
When only two sites of measurement are considered (e.g. BMD at lumbar spine and femoral neck), the relationship can be written
The last term of the above expression, P(A∩B), is the probability that both measurements are below the cut-off limit g. We assume that the scale of the measurements is standardised so their means and SDs are 0 and 1, respectively. If the correlation coefficient between the measurements is ρ, then the probability that both of two measurements, X and Z, are below the limit g and can be written
, where X and Y are independent and X has a normal distribution with the mean 0 and the standard deviation 1, and Y has a normal distribution with the mean 0 and the variance 1−ρ 2. Then the variance of ρ·X+Y is 1 and the covariance between X and ρ·X+Y is ρ, so the correlation coefficient is also ρ. Let Φ denote the standardised normal distribution function. We then obtain
and
Thus, the probability that any of the two measurements is below the limit g, P(A ∪ B) can be calculated by the two last-mentioned relationships.
Rights and permissions
About this article
Cite this article
Kanis, J.A., Johnell, O., Oden, A. et al. The use of multiple sites for the diagnosis of osteoporosis. Osteoporos Int 17, 527–534 (2006). https://doi.org/10.1007/s00198-005-0014-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-0014-9