Abstract
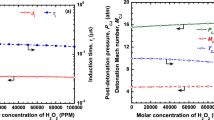
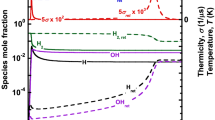
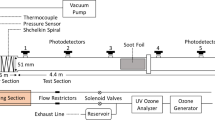
The unwarranted leakage/release of hydrogen gas from metal processing, automotive, petrochemical industries, and nuclear reactors, along with its subsequent ignition and transition to detonation, could lead to catastrophic damage to both life and property. The development of practical hazard prevention and safety control systems demands an understanding of the effectiveness of the chemical inhibitors to suppress/mitigate a detonation wave under varying operational conditions. In the current study, the inhibition efficiency of chemical inhibitors under varying mixture initial conditions was investigated using numerical computations. The inhibition efficiency of trifluoroiodomethane (CF\(_{\textrm{3}}\)I), carbon dioxide (CO\(_{\textrm{2}})\), and steam (H\(_{\textrm{2}}\)O) on hydrogen-oxygen/air mixtures was evaluated using a detailed chemical kinetic model for hydrogen oxidation. ZND computations were carried out over a range of initial mixture composition, pressure, and temperature. It was found that CF\(_{\textrm{3}}\)I is a better inhibitor than CO\(_{\textrm{2}}\) and H\(_{\textrm{2}}\)O at all the initial mixture conditions. However, at very high temperatures, the inhibitors CF\(_{\textrm{3}}\)I, CO\(_{\textrm{2}}\), and H\(_{\textrm{2}}\)O have a similar detonation inhibition efficiency. The inhibition efficiency of carbon dioxide and steam is comparable and significantly lower than CF\(_{\textrm{3}}\)I. The findings from the current work can be used to design optimized detonation safety systems over a range of practical operating conditions.









Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in the given article.
References
Wang, L., Ma, H., Shen, Z., Pan, J.: A comparative study of the explosion behaviors of H\(_{2}\) and C\(_{2}\)H\(_{4}\) with air, N\(_{2}\)O, and O\(_{2}\). Fire Saf. J. 119(1), 103260 (2021). https://doi.org/10.1016/j.firesaf.2020.103260
Crowl, D.A., Jo, Y.: The hazards and risks of hydrogen. J. Loss Prev. Process Ind. 20(1), 158–164 (2007). https://doi.org/10.1016/j.jlp.2007.02.002
Rosyid, O.A., Jablonski, D., Hauptmanns, U.: Risk analysis for the infrastructure of a hydrogen economy. Int. J. Hydrogen Energy 32(15), 3194–3200 (2007). https://doi.org/10.1016/j.ijhydene.2007.02.012
Thomas, J.K., Eastwood, C., Goodrich, M.: Are unconfined hydrogen vapor cloud explosions credible? Process Saf. Prog. 34(1), 36–43 (2014). https://doi.org/10.1002/prs.11685
Kim, W.K., Mogi, T., Dobashi, R.: Fundamental study on accidental explosion behavior of hydrogen-air mixtures in an open space. Int. J. Hydrogen Energy 38(19), 8024–8029 (2013). https://doi.org/10.1016/j.ijhydene.2013.03.101
Oran, E.S.: Understanding explosions - From catastrophic accidents to the creation of the universe. Proc. Combust. Inst. 35(1), 1–35 (2015). https://doi.org/10.1016/j.proci.2014.08.019
Oran, E.S., Gamezo, V.N.: Origins of the deflagration-to-detonation transition in gas-phase combustion. Combust. Flame 148(1–2), 4–47 (2007). https://doi.org/10.1016/j.combustflame.2006.07.010
Oran, E.S., Chamberlain, G., Pekalski, A.: Mechanisms and occurrence of detonations in vapor cloud explosions. Prog. Energy Combust. Sci. 77(1), 100804 (2020). https://doi.org/10.1016/j.pecs.2019.100804
Jiang, Y., Li, Y., Zhou, Y., Jiang, H., Zhang, K., Gao, Z., Gao, W.: Investigation on unconfined hydrogen cloud explosion with external turbulence. Int. J. Hydrogen Energy 47(13), 8658–8670 (2022). https://doi.org/10.1016/j.ijhydene.2021.12.167
Machniewski, P., Molga, E.: CFD analysis of large-scale hydrogen detonation and blast wave overpressure in partially confined spaces. Process Saf. Environ. Prot. 158(1), 537–546 (2022). https://doi.org/10.1016/j.psep.2021.12.032
Palacios, A., Bradley, D.: Hydrogen generation, and its venting from nuclear reactors. Fire Saf. J. 113(1), 102968 (2020). https://doi.org/10.1016/j.firesaf.2020.102968
Westbrook, C.K.: Inhibition of hydrocarbon oxidation in laminar flames and detonations by halogenated compounds. Proc. Combust. Inst. 19(1), 127–141 (1982). https://doi.org/10.1016/S0082-0784(82)80185-9
Van Tiggelen, P.J., Lefebvre, M.H.: Flame retardants effectiveness in gaseous detonation mitigation. Proceedings of Halon Options Technical Working Conference, Albuquerque, NM, May 12 –14 (1998)
Leclerc, B.F., Glaude, P.A., Come, G.M., Baronnet, F.: Inhibiting effect of CF\(_{\rm 3 }\)I on the reaction between CH4 and O2 in a jet-stirred reactor. Combust. Flame 103(3), 285–292 (1997). https://doi.org/10.1016/S0010-2180(96)00168-X
Mathieu, O., Goulier, J., Gourmel, F., Mannan, M.S., Chaumeix, N., Petersen, E.L.: Experimental study of the effect of CF\(_{\rm 3 }\)I addition on the ignition delay time and laminar flame speed of methane, ethylene, and propane. Proc. Combust. Inst. 35(3), 2731–2739 (2015). https://doi.org/10.1016/j.proci.2014.05.096
Moen, I.O., Ward, S.A., Thibault, P.A., Lee, J.H.S., Knystautas, R., Dean, T., Westbrook, C.K.: The influence of diluents and inhibitors on detonations. Proc. Combust. Inst. 20(1), 1717–1725 (1985). https://doi.org/10.1016/S0082-0784(85)80668-8
Zhang, C., Wen, J., Shen, X., Xiu, G.: Experimental study of hydrogen/air premixed flame propagation in a closed channel with inhibitions for safety consideration. Int. J. Hydrogen Energy 44(40), 22654–22660 (2019). https://doi.org/10.1016/j.ijhydene.2019.04.032
Kumar, D.S., Singh, A.V.: Inhibition of hydrogen-oxygen/air gaseous detonations using CF\(_{\rm 3}\)I, H\(_{\rm 2}\)O, and CO\(_{\rm 2 }\). Fire Saf. J. 124(1), 103405 (2021). https://doi.org/10.1016/j.firesaf.2021.103405
Drakon, A.V., Eremin, A.V., Mikheyeva, E.Y.: On chemical inhibition of shock wave ignition of hydrogen-oxygen mixtures. J. Phys: Conf. Ser. 946(1), 012062 (2018). https://doi.org/10.1088/1742-6596/946/1/012062
Evariste, F., Lefebre, M.H., Van Tiggelen, P.J.: Inhibition of detonation wave with halogenated compounds. Shock Waves 6(1), 233–239 (1996). https://doi.org/10.1007/BF02511380
Babushok, V., Noto, T., Burgess, D.R.F., Hamins, A., Tsang, W.: Influence of CF3I, CF3Br, and CF3H on the high-temperature combustion of methane. Combust. Flame 107(4), 351–367 (1996). https://doi.org/10.1016/S0010-2180(96)00052-1
Noto, T., Babushok, V., Hamins, A., Tsang, W.: Inhibition effectiveness of halogenated compounds. Combust. Flame 112(1–2), 147–160 (1998). https://doi.org/10.1016/S0010-2180(97)81763-4
Stamps, D.W., Tieszen, S.R.: The influence of initial pressure and temperature on hydrogen-air-diluent detonations. Combust. Flame 83(1), 353–364 (1991). https://doi.org/10.1016/0010-2180(91)90082-M
Lee, J.H.S.: The Detonation Phenomenon. Cambridge University Press, Cambridge (2008)
Vasil’ev, A.A.: Cell size as the main geometric parameter of a multifront detonation wave. J. Propul. Power 22(6), 1245–1260 (2012). https://doi.org/10.2514/1.20348
Tieszen, S.R., Stamps, D.W., Westbrook, C.K., Pitz, W.J.: Gaseous hydrocarbon-air detonations. Combust. Flame 84(3–4), 367–390 (1991). https://doi.org/10.1016/0010-2180(91)90013-2
Ciccarelli, G., Ginsberg, T., Boccio, J., Economos, C., Kinoshita, M.: Detonation cell size measurements and predictions in hydrogen-air-steam mixtures at elevated temperatures. Combust. Flame 99(2), 212–220 (1994). https://doi.org/10.1016/0010-2180(94)90124-4
Stamps, D.W., Slezak, S.E., Tieszen, S.R.: Observations of the cellular structure of fuel-air detonations. Combust. Flame 144(1–2), 289–298 (2006). https://doi.org/10.1016/j.combustflame.2005.06.016
Gavrikov, A.I., Efimenko, A.A., Dorofeev, S.B.: A model for detonation cell size prediction from chemical kinetics. Combust. Flame 120(1), 19–33 (2000). https://doi.org/10.1016/S0010-2180(99)00076-0
Shepherd, J.E.: Chemical kinetics of hydrogen-air-diluent detonations. Prog. Astronaut. Aeronaut. 106(1), 263–293 (1986). https://doi.org/10.2514/5.9781600865800.0263.0293
Westbrook, C.K., Urtiew, P.A.: Chemical kinetic prediction of critical parameters in gaseous detonations. Proc. Combust. Inst. 19(1), 615–623 (1982). https://doi.org/10.1016/S0082-0784(82)80236-1
Crane, J., Shi, X., Singh, A.V., Tao, Y., Wang, H.: Isolating the effect of induction length on detonation structure: Hydrogen-oxygen detonation promoted by ozone. Combust. Flame 200(1), 44–52 (2018). https://doi.org/10.1016/j.combustflame.2018.11.008
Ng, H., Ju, Y., Lee, J.: Assessment of detonation hazards in high-pressure hydrogen storage from chemical sensitivity analysis. Int. J. Hydrogen Energy 32(1), 93–99 (2007). https://doi.org/10.1016/j.ijhydene.2006.03.012
Mével, R., Lafosse, F., Catoire, L., Chaumeix, N., Dupré, G., Paillard, C.-E.: Induction delay times and detonation cell size prediction of hydrogen-nitrous oxide-diluent mixtures. Combust. Sci. Technol. 180(10–11), 1858–1875 (2008). https://doi.org/10.1080/00102200802261340
Zhang, B., Ng, H.D., Mével, R., Lee, J.H.S.: Critical energy for direct initiation of spherical detonations in H2/N2O/Ar mixtures. Int. J. Hydrogen Energy 36(1), 5707–5716 (2011). https://doi.org/10.1016/j.ijhydene.2011.01.175
Browne, S., Ziegler, J., Bitter, N.P., Schmidt, B.E., Lawson, J., Shepherd, J.E.: SDToolbox: Numerical Solution Methods for Shock and Detonation Jump Conditions. GALCIT Report FM2018.001, California Institute of Technology, Pasadena, CA (2023)
Goodwin, D.G., Moffat, H.K., Schoegl, I., Speth, R.L., Weber, B.W.: Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes, Version 3.0.0 (2023). https://www.cantera.org/
Smith, G.P., Tao, Y., Wang, H.: Foundational Fuel Chemistry Model Version 1.0 (FFCM-1) (2016). https://web.stanford.edu/group/haiwanglab/FFCM1/pages/FFCM1.html
Hastie, J.W.: Molecular basis of flame inhibition. J. Res. Natl Bureau Stand. Sect. A Phys. Chem. 77(6), 733–754 (1973). https://doi.org/10.6028/jres.077a.045
Acknowledgements
The financial support from the Aeronautics Research and Development Board (ARDB) is gratefully acknowledged for the current work (Grant no. ARDB/01/1042000 M/I). The authors would also like to acknowledge the financial support from the ISRO-IITK Space Technology Cell (Grant no. 2023664 G).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Ciccarelli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is based on work that was presented at the 29th International Colloquium on the Dynamics of Explosions and Reactive Systems (ICDERS), Siheung, Korea, July 23–28, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dahake, A., Singh, R.K. & Singh, A.V. Effect of initial conditions on the inhibition process of H\(_{\textrm{2}}\)–O\(_{\textrm{2}}\)/air detonations using CF\(_{\textrm{3}}\)I, CO\(_{\textrm{2}}\), and H\(_{\textrm{2}}\)O. Shock Waves (2024). https://doi.org/10.1007/s00193-024-01172-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00193-024-01172-7