Abstract
Introduction and hypothesis
Polypropylene meshes (PM) used in pelvic organ prolapse surgery are being withdrawn from the market. Although concerns about the usage of PMs in stress incontinence surgery have been raised, it is still one of the best methods of curing stress urinary incontinence. With advancements in stem cell-based therapies, especially mesenchymal stem cells (MSCs), it is believed that coating the synthetic meshes with MSCs may minimize excessive tissue reactions ultimately leading to clinical problems such as pain, erosion or extrusion of the implanted material. In our study we tried to show the possibility of coating the PM with placenta-derived MSCs.
Methods
Mesenchymal stem cells obtained from six placentas were isolated, cultured, and identified. MSCs were then soaked in either fibronectin or collagen prior to co-culturing with strips of PMs. One group is used as a control, and hence was not pretreated before co-culturing. Specimens were fixed and stained with both Gram and hematoxylin and eosin and marked with Vybran Dil and DAPI. All preparations were examined under a light microscope. The IMAGEJ program was utilized to determine the surface area of meshes coated with MSCs.
Results
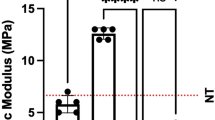
We clearly showed that PMs can be coated successfully with placenta-derived MSCs. The percentage of the coated area is significantly increased when meshes were pretreated with fibronectin or collagen (p<0.0001).
Conclusions
Placenta-derived MSCs can successfully coat PMs. The immunomodulatory properties of MSCs, which may be of great advantage in preventing the side effects of meshes, should be tested by in vivo and hopefully human studies before clinical applications.






Similar content being viewed by others
References
Seklehner S, Laudano MA, Xie D, Chughtai B, Lee RK. A meta-analysis of the performance of retropubic mid urethral slings versus transobturator mid urethral slings. J Urol. 2015;193(3):909–15.
Ashok K, Petri E. Failures and complications in pelvic floor surgery. World J Urol. 2012;30(4):487–94.
Barski D, Gerullis H, Georgas E, et al. Coating of mesh grafts for prolapse and urinary incontinence repair with autologous plasma: exploration stage of a surgical innovation. Biomed Res Int. 2014;2014:296–8.
Marinaro F, Sanchez-Margello FM, Alvarez V, et al. Meshes in a mess: mesenchymal stem cell-based therapies for soft tissue reinforcement. Acta Biomater. 2019;2019(85):60–74. https://doi.org/10.1016/j.actbio.2018.11.042.
Maytalman E, Yegani AA, Kozanoğlu I, Aksu F. Adrenergic receptor behaviors of mesenchymal stem cells obtained from different tissue sources and the effect of the receptor blockade on differentiation. J Recept Signal Transduct Res. 2022;42(4):349–60. https://doi.org/10.1080/10799893.2021.1957931.
Enes SR, Sjöland AA, Skog I, et al. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci Rep. 2016;6(6):29160. https://doi.org/10.1038/srep29160.
Karlsson H, Erkers T, Nava S, Ruhm S, Westgren M, Ringdén O. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol. 2012;167(3):543–55. https://doi.org/10.1111/j.1365-2249.2011.04540.x.
Dominici M, Le Blanc K, Slager-Cortenbach I, et al. Minimal criteria for defining multipotent mesenchymal stem cells. International Society for Cell Therapy Position statement. Cytotherapy. 2006;8(4):315–7.
Todros S, Pavan PG, Natali AN. Synthetic surgical meshes used in abdominal wall surgery. I. Materials and structural conformation. J Biomed Mater Res B Appl Biomater. 2017;105(3):689–99.
Sangster P, Morley R. Biomaterials in urinary incontinence and treatment of their complications. Indian J Urol. 2010;26(2):221–9.
Karlovsky ME, KushnerL BGH. Synthetic biomaterials for pelvic floor reconstruction. Curr Urol Rep. 2005;6(5):376–84.
Liang R, Knight K, Abramowitch S, Moalli PA. Exploring the basic science of prolapse meshes. Curr Opin Obstet Gynecol. 2016;28(5):413–9. https://doi.org/10.1097/GCO.0000000000000313.
Gigliobianco G, Regueros SR, Osman N, et al. Biomaterials for pelvic floor reconstructive surgery: how can we do better? Biomed Res Int. 2015;2015:968087. https://doi.org/10.1155/2015/968087.
Zou XH, Zhi YL, Chen X, Jin HM, Wang LL, Jiang YZ, Ouyang HW. Mesenchymal stem cell seeded knitted silk sling for the treatment of stress urinary incontinence. Biomaterials. 2010;31(18):4872–9.
Sima Y, Chen Y. Mesenchymal stem cell-based therapy in female pelvic floor disorders. Cell Biosci. 2020;10(10):104. https://doi.org/10.1186/s13578-020-00466-4.
Tran C, Damaser MS. The potential role of stem cells in the treatment of urinary incontinence. Ther Adv Urol. 2015;7(1):22–40. https://doi.org/10.1177/1756287214553968.
Marinaro F, Silva JM, Barros AA, et al. A fibrin coating method of polypropylene meshes enables the adhesion of menstrual blood-derived mesenchymal stromal cells: a new delivery strategy for stem cell-based therapies. Int J Mol Sci. 2021;22(24):13385. https://doi.org/10.3390/ijms222413385.
Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol. 2003;15(5):565–71.
Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418(3):491–506.
Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J. 2018;18(3):e264–77.
Mukherjee S, Darzi S, Rosamila A, et al. Blended nanostructured degradable mesh with endometrial mesenchymal stem cell promotes tissue integration and anti-inflammatory response in vivo for pelvic floor application. Biomacromolecules. 2019;20:454–68. https://doi.org/10.1021/acs.biomac.8b01661.
Acknowledgements
We would like to extend our gratitude to Biologist Hatice Irday, Biologist Sibel Erkoç and, Gülcin Daglioglu, MD, from the Central Laboratory of Çukurova University, Adana, Turkey, for their valuable support in flow cytometry analyses.
Funding
This study was funded by Alanya Alaaddin Keykubat University Scientific Research and Project Board (2021-04-03-MAP01).
Author information
Authors and Affiliations
Contributions
E. Aslan: project development, manuscript writing; E. Maytalman: data collection, laboratory experiments; D. Nemutlu Samur: data collection, laboratory experiments; E. Köle: data collection, manuscript writing; Ö.C. Günizi: data collection, laboratory experiments.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslan, E., Maytalman, E., Nemutlu Samur, D. et al. An in vitro pilot study investigating placenta-derived mesenchymal stem cell coating on polypropylene mesh materials. Int Urogynecol J 35, 553–559 (2024). https://doi.org/10.1007/s00192-023-05687-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05687-y