Abstract
Introduction and hypothesis
Sacral neuromodulation (SNM) has been established as an effective third-line therapy for non-obstructive urinary retention and urinary urgency-frequency syndrome. Device infection, ranging from 2–10%, is a severe complication usually necessitating device explanation. This study sought to demonstrate an infection protocol founded upon established device implantation risk factors and novel approaches to reduce the incidence of device infection, while maintaining good antibiotic stewardship following best practice statements.
Methods
A single-surgeon protocol was enacted from 2013 to 2022. Preoperatively, nasal swabs were cultured from each patient. If positive for methicillin-resistant Staphylococcus aureus or methicillin-sensitive Staphylococcus aureus, preoperative treatment with intranasal mupirocin was employed. Preoperative cefazolin was administered in patients with negative cultures or MSSA-positive. All protocol patients were given chlorhexidine wipes before surgery and prepped with a chlorhexidine scrub followed by alcohol/iodine paint. Post-procedural antibiotics were not given. Pre-protocol patients from 2011 to 2013 served as controls.
Results
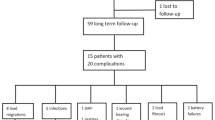
Pre-protocol (n = 87) patients had a significantly higher rate of device infection compared to protocol patients (n = 444) in both the percentage of patients experiencing device infection (4.6% vs 0.9%, p = 0.01) and percentage of procedures associated with device infection (2.9% vs 0.5%, p < 0.05). A successful culture of the nares was achieved in 91.4% of protocol patients, with 11.6% MRSA-positive. Risk ratio for infection of pre-protocol/protocol patients was 0.19 (0.05–0.77) with odds ratio 5.1 (1.3–20.0).
Conclusions
Utilization of a novel SNM infection protocol tailored to a patient’s preoperative MRSA colonization is associated with a reduction in the overall incidence of explant for device infection while avoiding prolonged postoperative antibiotic regimens.
Clinical trial registration
The study was initiated prior to January 18, 2017 and does not meet the definition of an applicable clinical trial (ACT) as defined in section 402 (J) of the US PHS Act.
Similar content being viewed by others
References
White WM, Mobley JD 3rd, Doggweiler R, Dobmeyer-Dittrich C, Klein FA. Incidence and predictors of complications with sacral neuromodulation. Urology. 2009;73(4):731–5. https://doi.org/10.1016/j.urology.2008.11.047.
Carmel ME, Vasavada SP, Goldman HB. Troubleshooting sacral neuromodulation issues. Curr Urol Rep. 2012;13(5):363–9. https://doi.org/10.1007/s11934-012-0268-7.
Wexner SD, Hull T, Edden Y, et al. Infection rates in a large investigational trial of sacral nerve stimulation for fecal incontinence. J Gastrointest Surg. 2010;14(7):1081–9. https://doi.org/10.1007/s11605-010-1177-z.
Mellgren A, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011;54(9):1065–75. https://doi.org/10.1097/DCR.0b013e31822155e9
Amundsen CL, Komesu YM, Chermansky C, et al. Two-year outcomes of sacral neuromodulation versus OnabotulinumtoxinA for refractory urgency urinary incontinence: a randomized trial. Eur Urol. 2018;74(1):66–73. https://doi.org/10.1016/j.eururo.2018.02.011.
Lee C, Pizarro-Berdichevsky J, Clifton MM, Vasavada SP. Sacral neuromodulation implant infection: risk factors and prevention. Curr Urol Rep. 2017;18(2):16. https://doi.org/10.1007/s11934-017-0663-1
Meurette G, Siproudhis L, Leroi AM, et al. Sacral neuromodulation with the InterStim system for faecal incontinence: results from a prospective French multicentre observational study. Color Dis. 2021;23(6):1463–73. https://doi.org/10.1111/codi.15507
Daniels DH, Powell CR, Braasch MR, Kreder KJ. Sacral neuromodulation in diabetic patients: success and complications in the treatment of voiding dysfunction. Neurourol Urodyn. 2010;29(4):578–81. https://doi.org/10.1002/nau.20791
Brueseke T, Livingston B, Warda H, Osann K, Noblett K. Risk factors for surgical site infection in patients undergoing sacral nerve modulation therapy. Female Pelvic Med Reconstr Surg. 2015;21(4):198–204. https://doi.org/10.1097/SPV.0000000000000183.
Noblett K, Lane F. Risk factors for infection following third-line therapy for overactive bladder. Current Bladder Dysfunction Reports. 2017;12(4):268–72.
Myer ENB, Petrikovets A, Slocum PD, et al. Risk factors for explantation due to infection after sacral neuromodulation: a multicenter retrospective case-control study. Am J Obstet Gynecol. 2018;219(1):78.e1–9. https://doi.org/10.1016/j.ajog.2018.04.005.
Haraway AM, Clemens JQ, He C, Stroup C, Atiemo HO, Cameron AP. Differences in sacral neuromodulation device infection rates based on preoperative antibiotic selection. Int Urogynecol J. 2013;24(12):2081–5. https://doi.org/10.1007/s00192-013-2121-z.
Noblett K, Benson K, Kreder K. Detailed analysis of adverse events and surgical interventions in a large prospective trial of sacral neuromodulation therapy for overactive bladder patients. Neurourol Urodyn. 2017;36(4):1136–9. https://doi.org/10.1002/nau.23076.
Guralnick ML, Benouni S, O'Connor RC, Edmiston C. Characteristics of infections in patients undergoing staged implantation for sacral nerve stimulation. Urology. 2007;69(6):1073–6. https://doi.org/10.1016/j.urology.2007.01.099.
Kessler TM, Burkhard FC, Madersbacher H, Kofler A, Poewe W, Kiss G. Safety of prolonged sacral neuromodulation tined lead testing. Curr Med Res Opin. 2008;24(2):343–7. https://doi.org/10.1185/030079908x253555.
Huwyler M, Kiss G, Burkhard FC, Madersbacher H, Kessler TM. Microbiological tined-lead examination: does prolonged sacral neuromodulation testing induce infection? BJU Int. 2009;104(5):646–50, dscussion 650. https://doi.org/10.1111/j.1464-410X.2009.08501.x.
Shahryarinejad A, Vardy MD, Filmar G, Walter ME, Kocher J. Sacral neuromodulator InterStim surgical site infection: two case reports. Female Pelvic Med Reconstr Surg. 2010;16(6):362–4. https://doi.org/10.1097/SPV.0b013e3181f5abe2.
Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA. 2015;313(21):2162–71. https://doi.org/10.1001/jama.2015.5387.
Weiser MC, Moucha CS. The current state of screening and decolonization for the prevention of Staphylococcus aureus surgical site infection after total hip and knee arthroplasty. J Bone Joint Surg Am. 2015;97(17):1449–58. https://doi.org/10.2106/JBJS.N.01114.
Harrison JL, Prendergast BD, Sandoe JAT. Guidelines for the diagnosis, management and prevention of implantable cardiac electronic device infection. Heart. 2015;101(4):250–2. https://doi.org/10.1136/heartjnl-2014-306873.
McConeghy KW, Mikolich DJ, LaPlante KL. Agents for the decolonization of methicillin-resistant Staphylococcus aureus. Pharmacotherapy. 2009;29(3):263–80. https://doi.org/10.1592/phco.29.3.263.
Forget V, Azzam O, Khouri C, Landelle C. What is the benefit of preoperative washing with chlorhexidine gluconate-impregnated cloths on the incidence of surgical site infections? A systematic review and meta-analysis. Infect Dis Now. 2022;52(4):185–92. https://doi.org/10.1016/j.idnow.2022.01.007
Eiselt D. Presurgical skin preparation with a novel 2% chlorhexidine gluconate cloth reduces rates of surgical site infection in orthopaedic surgical patients. Orthop Nurs. 2009;28(3):141–5. https://doi.org/10.1097/NOR.0b013e3181a469db.
Lightner DJ, Wymer K, Sanchez J, Kavoussi L. Best practice statement on urologic procedures and antimicrobial prophylaxis. J Urol Feb, 2020;203(2):351–6. https://doi.org/10.1097/ju.0000000000000509
Letzelter J, Hill J, Bradford H, Jacques. An overview of skin antiseptics used in orthopaedic surgery procedures. J Am Acad Orthop Surg. 2019:27(16):599–606
Deer TR, Provenzano DA, Hanes M, et al. The Neurostimulation appropriateness consensus committee (NACC) recommendations for infection prevention and management. Neuromodulation.2017;20(1):31–50. https://doi.org/10.1111/ner.12565
Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14(1):73–156. https://doi.org/10.1089/sur.2013.9999.
Lee EW, Lucioni A, Lee UJ, Kobashi KC. National Practice Patterns of infection prophylaxis for sacral neuromodulation device: a survey of high volume providers. Urology Practice. 2015;2(1):38–43. https://doi.org/10.1016/j.urpr.2014.07.003.
Author information
Authors and Affiliations
Contributions
Colin Goudelocke: protocol/project development, data collection and analysis, manuscript writing/editing
Hayden Hill: data collection, manuscript writing/editing
Nicholas Major: data collection, manuscript writing/editing
Anastasia Couvaras: data collection, manuscript writing/editing
Amy Long: data collection, manuscript editing
Corresponding author
Ethics declarations
Conflicts of interest
CG receives research support and speaker honoraria from Medtronic.
HH, NM, AC, and AL have no conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goudelocke, C., Hill, H., Major, N. et al. A novel sacral neuromodulation protocol is associated with reduction in removal for device infection. Int Urogynecol J 34, 2421–2428 (2023). https://doi.org/10.1007/s00192-023-05543-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05543-z