Abstract
Introduction and hypothesis
Mechanical trauma and oxidative injury are involved in the pathogenesis of stress urinary incontinence (SUI), and oxidative stress (OS) is considered a potential therapeutic target. The antioxidant properties of dimethyl fumarate (DMF), a potent activator of Nrf2, have been highlighted recently. We therefore predicted that DMF might have therapeutic effects on mechanical trauma-induced SUI.
Methods
The SUI mice model was established by vaginal distension (VD). Leak point pressure (LPP), serum OS biomarkers, cell proliferation and apoptosis, collagen, elastin, matrix metalloproteinases (MMP), Nrf2, the TGF-β1/Smad3 signaling pathway, and the associated tissue growth factors in the anterior vaginal wall were measured in either wild-type or Nrf2-knockout (Nrf2−/−) female C57BL/6 mice.
Results
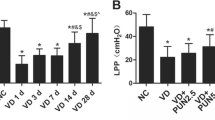
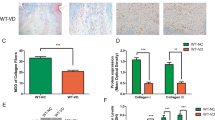
The results showed that DMF improved the VD-induced LPP reduction, alleviated oxidative injury, stimulated cell proliferation and inhibited apoptosis in the anterior vaginal wall tissue of mice. Moreover, DMF treatment reduced the hydrolysis of ECM proteins by MMP2 and MMP9. The above effects may be mediated by a series of tissue growth factors, including α-SMA, PAI-1, and TIMP-2, with the TGF-β1/Smad3 signaling pathway as the core regulatory mechanism. In further study, Nrf2−/− mice were used to replicate the SUI model. And the difference is that DMF failed to reactivate the TGF-β1/Smad3 pathway, nor did it improve LPP.
Conclusions
Dimethyl fumarate can ameliorate urethra closure dysfunction in the VD-induced SUI mice model, and the therapeutic effect of DMF is mediated by the Nrf2-dominated antioxidant system and its downstream TGF-β1/Smad3 signaling pathway.





Similar content being viewed by others
Abbreviations
- ARE:
-
Antioxidant response elements
- α-SMA:
-
Alfa-smooth muscle actin
- CAT:
-
Catalase activity
- CMS:
-
Cyclic mechanical strain
- DMF:
-
Dimethyl fumarate
- ECM:
-
Extracellular matrix
- Keap1:
-
Kelch-like ECH-associated protein 1
- LPP:
-
Leak point pressure
- MDA:
-
Malondialdehyde
- MMP:
-
Matrix metalloproteinase
- Nrf2:
-
Nuclear factor erythroid-2-related factor 2
- OS:
-
Oxidative stress
- PAI-1:
-
Plasminogen activator inhibitor-1
- PCNA:
-
Proliferating cell nuclear antigen
- ROS:
-
Reactive oxygen species
- SUI:
-
Stress urinary incontinence
- TGF-β1:
-
Transforming growth factor-beta1
- TIMP:
-
Tissue inhibitor of metalloproteinase
- tSOD:
-
Total superoxide dismutase
- VD:
-
Vaginal distension
References
Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary incontinence in women: a review. JAMA. 2017;318:1592–604. https://doi.org/10.1001/jama.2017.12137.
Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Primers. 2017;3:17042. https://doi.org/10.1038/nrdp.2017.42.
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of incontinence in the county of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–7.
Almousa S, Bandin VLA. The prevalence of urinary incontinence in nulliparous adolescent and middle-aged women and the associated risk factors: a systematic review. Maturitas. 2018;107:78–83. https://doi.org/10.1016/j.maturitas.2017.10.003.
Daly D, Clarke M, Begley C. Urinary incontinence in nulliparous women before and during pregnancy: prevalence, incidence, type, and risk factors. Int Urogynecol J. 2018;29:353–62. https://doi.org/10.1007/s00192-018-3554-1.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170(1713-20):1720–3. https://doi.org/10.1016/s0002-9378(94)70346-9.
Chen B, Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol. 2011;186:1768–72. https://doi.org/10.1016/j.juro.2011.06.054.
Tang J, Li B, Liu C, et al. Mechanism of mechanical trauma-induced extracellular matrix remodeling of fibroblasts in association with Nrf2/ARE signaling suppression mediating TGF-β 1/Smad3 signaling inhibition. Oxid Med Cell Longev. 2017;2017:1–14. https://doi.org/10.1155/2017/8524353.
Zeng Q, Guo Y, Liu Y, et al. Integrin-beta1, not integrin-beta5, mediates osteoblastic differentiation and ECM formation promoted by mechanical tensile strain. Biol Res. 2015;48:25. https://doi.org/10.1186/s40659-015-0014-y.
Chen YJ, Jeng JH, Chang HH, Huang MY, Tsai FF, Yao CC. Differential regulation of collagen, lysyl oxidase and MMP-2 in human periodontal ligament cells by low- and high-level mechanical stretching. J Periodontal Res. 2013;48:466–74. https://doi.org/10.1111/jre.12028.
Hinz B. The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. https://doi.org/10.1016/j.matbio.2015.05.006.
Sampson N, Berger P, Zenzmaier C. Redox signaling as a therapeutic target to inhibit myofibroblast activation in degenerative fibrotic disease. Biomed Res Int. 2014;2014:1–14. https://doi.org/10.1155/2014/131737.
Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–8. https://doi.org/10.1016/j.cellsig.2012.10.003.
Tang J, Liu C, Min J, Hu M, Li Y, Hong L. Potential therapeutic role of punicalagin against mechanical-trauma-induced stress urinary incontinence via upregulation of Nrf2 and TGF-beta1 signaling: effect of punicalagin on mechanical trauma induced SUI. Int Urogynecol J. 2017;28:947–55. https://doi.org/10.1007/s00192-017-3283-x.
Tang J, Liu C, Li B, et al. Protective role of nuclear factor Erythroid-2-related factor 2 against mechanical trauma-induced apoptosis in a vaginal distension-induced stress urinary incontinence mouse model. Oxid Med Cell Longev. 2019;2019:1–10. https://doi.org/10.1155/2019/2039856.
Li Q, Li B, Liu C, Wang L, Tang J, Hong L. Protective role of Nrf2 against mechanical-stretch-induced apoptosis in mouse fibroblasts: a potential therapeutic target of mechanical-trauma-induced stress urinary incontinence. Int Urogynecol J. 2018;29:1469–77. https://doi.org/10.1007/s00192-017-3545-7.
Linker RA, Haghikia A. Dimethyl fumarate in multiple sclerosis: latest developments, evidence and place in therapy. Ther Adv Chronic Dis. 2016;7:198–207. https://doi.org/10.1177/2040622316653307.
Kornberg MD, Bhargava P, Kim PM, et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 2018;360:449–53. https://doi.org/10.1126/science.aan4665.
Saidu N, Kavian N, Leroy K, Jacob C, Nicco C, Batteux F, Alexandre J. Dimethyl fumarate, a two-edged drug: current status and future directions. Med Res Rev. 2019;39(5):1923–52. https://doi.org/10.1002/med.21567.
Belcher JD, Chen C, Nguyen J, et al. Control of oxidative stress and inflammation in sickle cell disease with the nrf2 activator dimethyl fumarate. Antioxid Redox Sign. 2017;26:748–62. https://doi.org/10.1089/ars.2015.6571.
Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A. 2016;113:4777–82. https://doi.org/10.1073/pnas.1603907113.
Huang Y, Daneshgari F, Liu G. Successful induction of stress urinary incontinence in mice by vaginal distension does not depend on the estrous cycle. Urology. 2014;83:951–8. https://doi.org/10.1016/j.urology.2013.12.028.
Chen H, Chen C, Lin Y, Chen Y, Chen W, Chen C. Proteomic analysis related to stress urinary incontinence following vaginal trauma in female mice. Eur J Obstet Gynecol Reprod Biol. 2013;171:171–9. https://doi.org/10.1016/j.ejogrb.2013.08.034.
Hijaz A, Daneshgari F, Sievert K, Damaser MS. Animal models of female stress urinary incontinence. J Urol. 2008;179:2103–10. https://doi.org/10.1016/j.juro.2008.01.096.
Herrera-Imbroda B, Lara MF, Izeta A, Sievert K, Hart ML. Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv Drug Deliver Rev. 2015;82-83:106–16. https://doi.org/10.1016/j.addr.2014.10.018.
Huang J, Cheng M, Ding Y, Chen L, Hua K. Modified vaginal dilation rat model for postpartum stress urinary incontinence. J Obstet Gynaecol Res. 2013;39:256–63. https://doi.org/10.1111/j.1447-0756.2012.01959.x.
Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol. 2005;98:1884–90. https://doi.org/10.1152/japplphysiol.01071.2004.
Woo LL, Hijaz A, Kuang M, Penn MS, Damaser MS, Rackley RR. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177:1568–72. https://doi.org/10.1016/j.juro.2006.11.047.
Nomiya M, Andersson KE, Yamaguchi O. Chronic bladder ischemia and oxidative stress: new pharmacotherapeutic targets for lower urinary tract symptoms. Int J Urol. 2015;22:40–6. https://doi.org/10.1111/iju.12652.
Acknowledgements
The authors would like to thank all the teachers in the Department of Gynecology and Obstetrics, Central Laboratory and Experimental Animal Center of Renmin Hospital of Wuhan University for their assistance. Thanks to the National Natural Science Foundation of China for funding.
Funding
This study was funded by the National Natural Science Foundation of China (grant no: 81701424).
Author information
Authors and Affiliations
Contributions
Cheng Liu: investigation, data analysis, manuscript writing; Ying Wang: investigation, data analysis, manuscript writing; Yang Li: investigation, data collection; Jianming Tang: methodology; Shasha Hong: data collection; Li Hong: project development.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, C., Wang, Y., Li, Y. et al. Dimethyl fumarate ameliorates stress urinary incontinence by reversing ECM remodeling via the Nrf2-TGF-β1/Smad3 pathway in mice. Int Urogynecol J 33, 1231–1242 (2022). https://doi.org/10.1007/s00192-021-05061-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-05061-w