Abstract
Purpose
Storage-phase bladder dysfunction can develop after pelvic radiotherapy. As the alpha-1d adrenoreceptor (a1d-AR) is dominant in the human detrusor, we aimed to investigate the effect of an a1d-AR antagonist on bladder dysfunction after pelvic radiotherapy in a rat model.
Materials and methods
Twenty-four female Wistar rats were used. Eight rats (14–15 weeks, 250–300 g) were randomized to three groups (normal reference group, radiation alone group and radiation plus naftopidil group). An 18-Gy dose of radiotherapy was applied to the radiation alone and radiation plus naftopidil groups. Naftopidil (20 mg/kg) was administered daily to the radiation plus naftopidil group. Four weeks after radiation, all rats underwent cystometry and were killed for reverse transcription polymerase chain reaction to detect mRNAs [a1d-AR, brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF)], Western blot to detect proteins (a1d-AR, extracellular-signal-regulated kinase, BDNF and VEGF) and immunohistochemistry.
Results
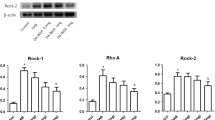
Compared to the radiation alone group, (1) the decrease in the mRNA and protein expression of a1d-AR and VEGF was ameliorated, (2) the increase in the expression of BDNF mRNA and proteins such as extracellular-signal-regulated kinase and BDNF was suppressed, (3) submucosal thickness and vascularity on immunohistochemistry were improved, and (4) the baseline intravesical pressure and intercontraction interval in cystometry were ameliorated in the radiation plus naftopidil group.
Conclusion
Administration of an a1d-AR antagonist could improve storage-phase bladder dysfunction after radiotherapy not only by upregulating a1d-AR, which might decrease bladder compliance, but also by enhancing vascularity, which might protect the urinary bladder from chronic ischemic inflammation.




Similar content being viewed by others
References
Katepratoom C, Manchana T, Amornwichet N. Lower urinary tract dysfunction and quality of life in cervical cancer survivors after concurrent chemoradiation versus radical hysterectomy. Int Urogynecol J. 2014;25:91–6. https://doi.org/10.1007/s00192-013-2151-6.
Lepor H, Baumann M, Shapiro E. The alpha adrenergic binding properties of terazosin in the human prostate adenoma and canine brain. J Urol. 1988;140:664–7. https://doi.org/10.1016/s0022-5347(17)41751-4.
Nasu K, Moriyama N, Kawabe K, et al. Quantification and distribution of alpha 1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br J Pharmacol. 1996;119:797–803. https://doi.org/10.1111/j.1476-5381.1996.tb15742.x.
Malloy BJ, Price DT, Price RR, et al. Alpha1-adrenergic receptor subtypes in human detrusor. J Urol. 1998;160:937–43. https://doi.org/10.1097/00005392-199809010-00092.
Schwinn D, Price DT, Narayan P. α1-Adrenoceptor subtype selectivity and lower urinary tract symptoms. Mayo Clin Proc. 2004;79:1423–34. https://doi.org/10.4065/79.11.1423.
Sugaya K, Nishijima S, Miyazato M, et al. Effects of intrathecal injection of tamsulosin and naftopidil, alpha-1A and -1D adrenergic receptor antagonists, on bladder activity in rats. Neurosci Lett. 2002;328:74–6. https://doi.org/10.1016/s0304-3940(02)00459-7.
Hara N, Mizusawa T, Obara K, Takahashi K. The role of naftopidil in the management of benign prostatic hyperplasia. Ther Adv Urol. 2013;5:111–9. https://doi.org/10.1177/1756287212461681.
Nishino Y, Masue T, Miwa K, et al. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006;97:747–51, discussion 51. https://doi.org/10.1111/j.1464-410X.2006.06030.x.
Yamaguchi S, Osanai H, Numata A, et al. alpha1D/A-adrenoceptor antagonist naftopidil for the male lower urinary tract symptoms associated with benign prostatic hyperplasia: efficacy of dose increase therapy. Int J Urol. 2013;20:513–9. https://doi.org/10.1111/j.1442-2042.2012.03188.x.
Takeda M, Homma Y, Araki I, et al. Predictive factors for the effect of the alpha1-D/a adrenoceptor antagonist naftopidil on subjective and objective criteria in patients with neurogenic lower urinary tract dysfunction. BJU Int. 2011;108:100–7. https://doi.org/10.1111/j.1464-410X.2010.09682.x.
Hampel C, Dolber PC, Smith MP, et al. Modulation of bladder alpha1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002;167:1513–21. https://doi.org/10.1016/S0022-5347(05)65355-4.
Dmitrieva N, Zhang G, Nagabukuro H. Increased alpha1D adrenergic receptor activity and protein expression in the urinary bladder of aged rats. World J Urol. 2008;26:649–55. https://doi.org/10.1007/s00345-008-0292-x10.1007/s00345-008-0292-x.
Lee G, Park H, Park SH, Lee JG. Modulation of alpha 1 adrenergic receptors on urinary bladder in rat spinal cord injury model. Int Neurourol J. 2012;16:62–8. https://doi.org/10.5213/inj.2012.16.2.62.
Majima T, Yamamoto T, Funahashi Y. Effect of Naftopidil on bladder microcirculation in a rat model of bladder outlet obstruction. LUTS. 2017;9:111–6. https://doi.org/10.1111/luts.12119.
Ochodnicky P, Cruz CD, Yoshimura N, et al. Neurotrophins as regulators of urinary bladder function. Nat Rev Urol. 2012;9:628–37. https://doi.org/10.1038/nrurol.2012.178.
Jiang HH, Song QX, Gill BC, et al. Electrical stimulation of the pudendal nerve promotes neuroregeneration and functional recovery from stress urinary incontinence in a rat model. Am J Physiol Renal Physiol. 2018;315:F1555–F64. https://doi.org/10.1152/ajprenal.00431.2017.
Deng Z, Sui G, Rosa PM, Zhao W. Radiation-induced c-Jun activation depends on MEK1-ERK1/2 Signaling pathway in microglial cells. PLoS One. 2012;7:e36739. https://doi.org/10.1371/journal.pone.0036739.
Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. https://doi.org/10.1371/journal.pbio.1000412.
Okazaki S, Murata K, Noda SE, et al. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J Radiat Res. 2019;60:490–500. https://doi.org/10.1093/jrr/rrz023.
Toita T, Kakinohana Y, Ogawa K, et al. Combination external beam radiotherapy and high-dose-rate intracavitary brachytherapy for uterine cervical cancer: analysis of dose and fractionation schedule. Int J Radiat Oncol Biol Phys. 2003;56:1344–53. https://doi.org/10.1016/s0360-3016(03)00288-8.
Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–68. https://doi.org/10.1259/bjr/31372149.
Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. https://doi.org/10.1259/0007-1285-62-740-679.
Institutional Animal Care and Use Committee. Anesthesia (Guideline). 2019. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia. Accessed Nov 19 2019.
Yamashita S, Katsumata O. Heat-induced antigen retrieval in immunohistochemistry: mechanisms and applications. Methods Mol Biol. 2017;1560:147–61. https://doi.org/10.1007/978-1-4939-6788-9_10.
Lee DS, Kim SJ. Kwon EB, et al comparison of in vivo biocompatibilities between parylene-C and polydimethylsiloxane for implantable microelectronic devices. Bull Mater Sci. 2013;36:1127–32 https://www.ias.ac.in/public/Volumes/boms/036/06/1127-1132.pdf.
Elrashidy RA, Liu G. Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp Mol Pathol. 2019;111:104304. https://doi.org/10.1016/j.yexmp.2019.104304.
Lee JY, Park JM, Na YG, et al. Expression of bladder α1-adrenoceptor subtype after relief of partial bladder outlet obstruction in a rat model. Investig Clin Urol. 2020;61:297–303. https://doi.org/10.4111/icu.2020.61.3.297.
Park MG, Park HS, Lee JG, Kim HJ. Changes in awake Cystometry and expression of bladder β-adrenoceptors after partial bladder outlet obstruction in male rats. Int Neurourol J. 2010;14:157–63. https://doi.org/10.5213/inj.2010.14.3.157.
Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. https://doi.org/10.1093/ilar.47.1.5.
Kojima Y, Sasaki S, Kubota Y, et al. Up-regulation of alpha1a and alpha1d-adrenoceptors in the prostate by administration of subtype selective alpha1-adrenoceptor antagonist tamsulosin in patients with benign prostatic hyperplasia. J Urol. 2011;186:1530–6. https://doi.org/10.1016/j.juro.2011.05.048.
Lepor H. Long-term evaluation of Tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Tamsulosin Invest Group Urol. 1998;51:901–6. https://doi.org/10.1016/s0090-4295(98)00127-7.
Smit SG, Heyns CF. Management of radiation cystitis. Nat Rev Urol. 2010;7:206–14. https://doi.org/10.1038/nrurol.2010.23.
Ikeda Y, Zabbarova IV, Birder LA, et al. Relaxin-2 therapy reverses radiation-induced fibrosis and restores bladder function in mice. Neurourol Urodyn. 2018;37:2441–51. https://doi.org/10.1002/nau.23721.
Sekerci CA, Tanidir Y, Toprak T, et al. Value of urinary brain-derived neurotrophic factor levels on the assessment of botulinum toxin type a treatment for neurogenic detrusor overactivity in children with myelodysplasia. J Urol. 2019;201:174–80. https://doi.org/10.1016/j.juro.2018.06.065.
Minet E, Arnould T, Michel G, et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–8. https://doi.org/10.1016/s0014-5793(00)01181-9.
Perez-Aso M, Segura V, Montó F, et al. The three α1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation. Biochim Biophys Acta. 1833;2013:2322–33. https://doi.org/10.1016/j.bbamcr.2013.06.013.
Hague C, Gonzalez-Cabrera PJ, Jeffries WB, Abel PW. Relationship between α1-adrenergic receptor-induced contraction and extracellular signal-regulated kinase activation in the bovine inferior alveolar artery. J Pharmacol Exp Ther. 2002;303:403–11. https://doi.org/10.1124/jpet.102.037531.
Yamamoto H, Rundqvist H, Branco C, et al. Autocrine VEGF isoforms differentially regulate endothelial cell behavior. Front Cell Dev Biol. 2016;4:99. https://doi.org/10.3389/fcell.2016.00099.
Golubeva AV, Zhdanov AV, Mallel G, et al. The mouse cyclophosphamide model of bladder pain syndrome: tissue characterization, immune profiling, and relationship to metabotropic glutamate receptors. Physiol Rep. 2014;2:e00260. https://doi.org/10.1002/phy2.260.
Gordon IO, Agrawal N, Willis E, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922–39. https://doi.org/10.1111/apt.14526.
Acknowledgements
We are particularly grateful to Jong-Hoon Lee in the Department of Radiation Oncology for providing us with the opportunity to perform this study. We are also grateful to Inae Park (Department of Urology, St. Vincent’s Hospital) and Eun Yong Shin (The Institute of Medical Science, St. Vincent’s Hospital) for assisting with all of the experiments. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C2915) and by a grant from Korea Research Foundation for Gynecologic Cancer.
Author information
Authors and Affiliations
Contributions
Protocol/project development: DS Lee; Data collection: DS Lee and HY Kim; Data analysis: SJ Lee and DS Lee; Manuscript writing/editing: SJ Lee and DS Lee; Performing experiment: DS Lee and HY Kim.
Corresponding author
Ethics declarations
All experimental protocols were approved by the Institutional Animal Care and Use Committee of St. Vincent’s Hospital, The Catholic University of Korea (approval no. IACUC 17-1; date: 26 January 2017).
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lee, S.J., Kim, H.Y. & Lee, D.S. Effects of an alpha-1d adrenoreceptor antagonist (naftopidil) on bladder dysfunction after radiotherapy in female rats. Int Urogynecol J 32, 2747–2755 (2021). https://doi.org/10.1007/s00192-020-04472-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04472-5