Abstract
Introduction and hypothesis
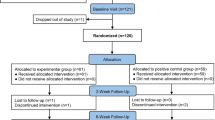
We present the design of a randomized controlled trial, Fluids Affecting Bladder Urgency and Lower Urinary Symptoms (FABULUS), with the purpose of testing the common clinical advice of treating overactive bladder by eliminating potentially irritating beverages (PIBs) that are caffeinated, artificially sweetened, citric, or alcoholic. The primary hypothesis is that women taught to reduce PIBs will show less void frequency compared with a control group instructed in diet/exercise recommendations. Secondary outcomes include change in urgency symptoms and volume per void.
Methods
We report the methods for FABULUS and discuss how challenges presented in the literature and from a prior proof-of-concept feasibility trial are addressed by strengthening study design, procedures, and instruments. We introduce the concept of standardized automated tutorials for assisting participants in compliance from study start to finish. The tutorials contain a detailed explanation of the study, including tips for complying with the extensive diary requirements, and parallel tutorials to intervention and control groups for consistency in format and time of instructional content. The intervention tutorial on eliminating PIBs places emphasis on maintaining steady fluid intake volume, as fluctuations have been a confounder in prior work.
Results
Study results promise to inform about both the tutorial approach and specific PIB reduction for effectively treating overactive bladder.
Conclusions
OAB can have a negative impact on quality of life, and current medical treatments carry costs and side-effect risks. If simple lifestyle changes can improve or prevent these bladder symptoms, multiple medical and public health advances could result.


Similar content being viewed by others
References
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. https://doi.org/10.1002/nau.20798.
Coyne KS, Sexton CC, Bell JA, Thompson CL, Dmochowski R, Bavendam T, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn. 2013;32(3):230–7. https://doi.org/10.1002/nau.22295.
Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80(1):90–6. https://doi.org/10.1016/j.urology.2012.04.004.
Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011;65(5):567–85. https://doi.org/10.1111/j.1742-1241.2010.02626.x.
Visco AG, Zyczynski H, Brubaker L, Nygaard I, Xu X, Lukacz ES, et al. Cost-effectiveness analysis of anticholinergics versus Botox for urgency urinary incontinence: results from the anticholinergic versus Botox comparison randomized trial. Female Pelvic Med Reconstr Surg. 2016;22(5):311–6. https://doi.org/10.1097/SPV.0000000000000277.
Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–7. https://doi.org/10.1001/jamainternmed.2014.7663.
Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721–32. https://doi.org/10.1001/jamaneurol.2016.0580.
Bradley CS, Erickson BA, Messersmith EE, Pelletier-Cameron A, Lai HH, Kreder KJ, et al. Evidence of the impact of diet, fluid intake, caffeine, alcohol and tobacco on lower urinary tract symptoms: a systematic review. J Urol. 2017;198(5):1010–20. https://doi.org/10.1016/j.juro.2017.04.097.
Miller JM, Garcia CE, Hortsch SB, Guo Y, Schimpf MO. Does instruction to eliminate coffee, tea, alcohol, carbonated, and artificially sweetened beverages improve lower urinary tract symptoms?: a prospective trial. J Wound Ostomy Continence Nurs. 2016;43(1):69–79. https://doi.org/10.1097/WON.0000000000000197.
Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005;174(1):187–9. https://doi.org/10.1097/01.ju.0000162020.10447.31.
Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102(1):62–6. https://doi.org/10.1111/j.1464-410X.2008.07463.x.
Bower WF, Moore KH, Adams RD. A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J Urol. 2001;166(6):2420–2.
Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–74.
De Wachter S, Wyndaele JJ. Frequency-volume charts: a tool to evaluate bladder sensation. Neurourol Urodyn. 2003;22(7):638–42. https://doi.org/10.1002/nau.10160.
Schimpf MO, Patel M, O’Sullivan DM, Tulikangas PK. Difference in quality of life in women with urge urinary incontinence compared to women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(7):781–6. https://doi.org/10.1007/s00192-009-0855-4.
Cameron AP, Wiseman JB, Smith AR, Merion RM, Gillespie BW, Bradley CS, et al. Are three-day voiding diaries feasible and reliable? Results from the symptoms of lower urinary tract dysfunction research network (LURN) cohort. Neurourol Urodyn. 2019;38(8):2185–93. https://doi.org/10.1002/nau.24113.
Acknowledgements
The authors thank Sarah Block for her assistance with manuscript preparation, Caroline Garcia for her assistance with producing the tutorials, and study staff Ruta Misiunas and Meg Tolbert for recruitment and data collection.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Pfizer Global Investigator-Initiated Research Grant (grant no. GA6120A8) and Blue Cross Blue Shield of Michigan Foundation, Investigator-Initiated Grant (grant no. 002607.II).
Author information
Authors and Affiliations
Contributions
MO Schimpf: Protocol/project development, Data collection/management, Data analysis, Manuscript writing/editing.
AR Smith: Protocol/project development, Data collection/management, Data analysis, Manuscript writing/editing.
JM Miller: Protocol/project development, Data collection/management, Data analysis, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schimpf, M.O., Smith, A.R. & Miller, J.M. Fluids affecting bladder urgency and lower urinary symptoms (FABULUS): methods and protocol for a randomized controlled trial. Int Urogynecol J 31, 1033–1040 (2020). https://doi.org/10.1007/s00192-019-04209-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-04209-z