Abstract
Introduction and hypothesis
Surgical repair of pelvic organ prolapse often includes native tissue repair during which the patient’s own vaginal connective tissue is used to achieve pelvic support. This method, based on plication and suspension often yields suboptimal anatomical outcomes, possibly due to inadequate healing of the vaginal connective tissue. We hypothesized that age might have a negative effect on the time course and tissue biomechanics of vaginal wound healing in a rat model.
Methods
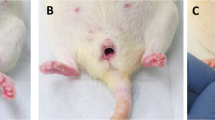
Fifty young (12 weeks) and old (12 months) female 344BN Fischer rats were subjected to a posterior midline vaginal incision. The time course of repair was determined by measuring the size of the wound on days 1, 3, 7, and 14 post-injury. These findings correlated with the immune response to injury using a marker of impaired wound healing, the inflammatory cytokine macrophage migration inhibitory factor in the vaginal muscularis. Biomechanical properties of the healed vaginal tissue were tested 30 days post-injury.
Results
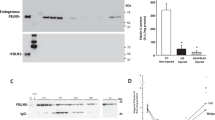
Wound healing was assessed on days 1, 3, 7, and 14 post-injury. On day 3 post-injury, the wounds in the young animals had all closed whereas the wounds in the old animals remained open. Furthermore, on day 7, the wound gap was still filled with granulation tissue in the old rats, whereas for the young rats, the wound area was almost indistinguishable from the non-injured area. Macrophage migration inhibitory factor was highly expressed in the vaginal epithelium and in the vaginal muscularis after injury. When compared with young animals, macrophage migration inhibitory factor levels of old rats began to rise more than 2 days later and the increased tissue expression persisted for 7 days longer. The breakpoint force of the healed vagina of old rats was almost 4-fold weaker than in young rats. At 30 days post-injury, the healed vagina in old rats regained less of the original (healthy) force at breakpoint than the young rats.
Conclusions
In this rat model, age impaired vaginal wound healing, which was reflected in the altered inflammatory response to injury and reduced tissue strength.




Similar content being viewed by others
References
Nygaard I, Barber MD, Burgio KL, et al. Pelvic floor disorders network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–6.
Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278–83.
Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg. 2003;27:54–61.
Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3(6):337–45.
Hardman MJ, Waite A, Zeef L, Burow M, Toshinori N, Ashcroft GS. Macrophage migration inhibitory factor—a central regulator of wound healing. Am J Pathol. 2005;167(6):1561–74.
Ashcroft GS, Horan MA, Ferguson MWJ. The effects of ageing on wound healing: immunolocalisation of growth factors and their receptors in a murine incisional model. J Anat. 1997;190 (Pt. 3):351–65.
Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–2.
Abe R, Shimizu T, Ohkawara A, Nishihira J. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochim Biophys Acta. 2000;1500:1–9.
Zhao Y, Shimizu T, Nishihira J, et al. Tissue regeneration using macrophage migration inhibitory factor-impregnated gelatin microbeads in cutaneous wounds. Am J Pathol. 2005;167(6):1519–29.
Alperin M, Feola A, Meyn L, Duerr R, Abramowitch S, Moalli P. Collagen scaffold: a treatment for simulated maternal birth injury in the rat model. Am J Obstet Gynecol. 2010;202(6):589.e1–8.
McPherson JC 3rd, Costoff A, Mahesh VB. Effects of aging on the hypothalamic-hypophyseal-gonadal axis in female rats. Fertil Steril. 1977;28(12):1365–70.
Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978 Nov;103(5):1855–9.
Ashcroft GS, Horan MA, Ferguson MWJ. Aging is associated with reduced deposition of specific extracellular matrix components, an upregulation of angiogenesis, and an altered inflammatory response in a murine incisional wound healing model. J Invest Dermatol. 1997;108:430–7.
Ashcroft GS, Mills SJ, Lei K, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–18.
Karp DR, Jean-Michel M, Johnston Y, Suciu G, Aguilar VC, Davila GW. A randomized clinical trial of the impact of local estrogen on postoperative tissue quality after vaginal reconstructive surgery. Female Pelvic Med Reconstr Surg. 2012;18(4):211–5.
Ripperda CM, Maldonado PA, Acevedo JF, et al. Vaginal estrogen: a dual-edged sword in postoperative healing of the vaginal wall. Menopause. 2017;24(7):838–49.
Abramov Y, Webb AR, Miller JJ, et al. Biomechanical characterization of vaginal versus abdominal surgical wound healing in the rabbit. Am J Obstet Gynecol. 2006;194(5):1472–7.
Funding
This work was supported by the following NIH grant: NICHD U10-HD047890 (DS and KS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Disclaimer
The findings and conclusions in this paper have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy. The mention of commercial products, their sources, or their use in connection with material reported herein, is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
(JPG 28 kb)
Rights and permissions
About this article
Cite this article
Shveiky, D., Iglesia, C.B., Sarkar Das, S. et al. Age-associated impairments in tissue strength and immune response in a rat vaginal injury model. Int Urogynecol J 31, 1435–1441 (2020). https://doi.org/10.1007/s00192-019-04008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-04008-6