Abstract
Introduction and hypothesis
The aim of the present systematic review and meta-analysis was to assess the effectiveness and safety of injections of the new bulking agent Urolastic® in the treatment of patients with stress urinary incontinence (SUI).
Methods
A systematic search was carried out to select observational and experimental studies on Urolastic® in female patients with SUI. Three different databases, Pubmed, the Cochrane Central Register of Controlled Trials, and Scopus, were used to retrieve scientific articles published from their inception to 31 January 2018.
Results
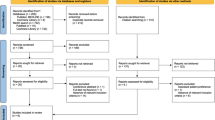
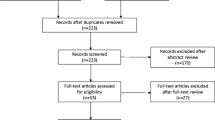
Eight full texts were evaluated but only five were selected for the qualitative and quantitative analyses. Duration of follow-up after Urolastic® injections was significantly heterogeneous, ranging from 6 to 24 months. Secondary injections were needed in 16.7%–35.0% of the treated patients. The pooled proportion of secondary injections was 20% (95% CI: 15%–24%; I2: 0%). Subjective improvement, measured by different means (i.e., patient global impression of improvement PGI-I score) was only assessed by 40% of the selected papers and was > 80% in two cohorts. The objective treatment success was evaluated by four (80.0%) papers and was achieved in all cohorts with a wide proportional range: from 32.7% (i.e., patients without objective SUI symptom cough tests and with a negative pad test) to 67.0%. Its pooled proportion was 57% (95% CI: 38%–75%; I2: 82.3%).
Conclusions
Urolastic® showed effectiveness in patients with SUI during a follow-up period of 6–24 months.





Similar content being viewed by others
References
Tantanasis T, Daniilidis A, Pantelis A, Chatzis P, Vrachnis N. Minimally invasive techniques for female stress urinary incontinence, how, why. when Arch Gynecol Obstet. 2013;288(5):995–1001.
Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjälmås K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62(4 Suppl 1):16–23.
Patel DA, Xu X, Thomason AD, Ransom SB, Ivy JS, DeLancey JO. Childbirth and pelvic floor dysfunction: an epidemiologic approach to the assessment of prevention opportunities at delivery. Am J Obstet Gynecol. 2006;195(1):23–8.
Alas AN, Chinthakanan O, Espaillat L, Plowright L, Davila GW, Aguilar VC. De novo stress urinary incontinence after pelvic organ prolapse surgery in women without occult incontinence. Int Urogynecol J. 2017;28(4):583–90.
Capobianco G, Wenger JM, Meloni GB, Dessole M, Cherchi PL, Dessole S. Triple therapy with lactobacilli acidophili, estriol plus pelvic floor rehabilitation for symptoms of urogenital aging in postmenopausal women. Arch Gynecol Obstet. 2014;289(3):601–8.
Dumoulin C, Hay-Smith EJ, Mac Habee-Seguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;(5):CD005654.
Urinary Incontinence in Women: the Management of Urinary Incontinence in Women: NICE Clinical Guideline CG171, National Institute for Health and Care Excellence, London (UK), 2013 Available: http://guidance.nice.org.uk/CG171.
Capobianco G, Madonia M, Morelli S, Dessole F, De Vita D, Cherchi PL, Dessole S. Management of female stress urinary incontinence: a care pathway and update. Maturitas 2018; 109: 32–38.
Kurien A, Narang S, Han HC. Tension-free vaginal tape-Abbrevo procedure for female stress urinary incontinence: a prospective analysis over 22 months. Singap Med J. 2017;58(6):338–42.
Capobianco G, Dessole M, Lutzoni R, Surico D, Ambrosini G, Dessole S. TVT-ABBREVO: efficacy and two years follow-up for the treatment of stress urinary incontinence. Clin Exp Obstet Gynecol. 2014;41(4):445–7.
American Urological Association. Incontinence 2017. https://www.auanet.org/education/guidelines/incontinence.cfm.
Mamut A, Carlson KV. Periurethral bulking agents for female stress urinary incontinence in Canada. Can Urol Assoc J. 2017 Jun;11(6 Suppl 2):S152–4.
Herschorn S. Injection therapy for urinary incontinence. In: Wein A, Kavoussi LR, Novick AC, et al., editors. Campbell-Walsh urology. 11th ed. Philadelphia: Saunders; 2013. p. 2049–69.
Kotb AF, Campeau L, Corcos J. Urethral bulking agents: techniques and outcomes. Curr Urol Rep. 2009;10:396–400.
Kowalik CR, Casteleijn FM, van Eijndhoven HWF, Zwolsman SE, Roovers JWR. Results of an innovative bulking agent in patients with stress urinary incontinence who are not optimal candidates for mid-urethral sling surgery. Neurourol Urodyn 2018;37(1):339–345.
Renard J, Citeri M, Zanollo L, Guerrer C, Rizzato L, Frediani L, et al. Treatment of stress urinary incontinence in neurological patients with an injectable elastomer prosthesis: preliminary results. Int Neurourol J. 2017;21(1):75–9.
de Vries AM, van Breda HMK, Fernandes JG, Venema PL, Heesakkers JPFA. Para-urethral injections with Urolastic® for treatment of female stress urinary incontinence: subjective improvement and safety. Urol Int. 2017;99(1):91–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Zajda J, Farag F. Urolastic for the treatment of women with stress urinary incontinence: 24-month follow-up. Cent European J Urol. 2015;68(3):334–8.
Futyma K, Miotła P, Gałczyński K, Baranowski W, Doniec J, Wodzisławska A, et al. An open multicenter study of clinical efficacy and safety of Urolastic, an injectable implant for the treatment of stress urinary incontinence: one-year observation. Biomed Res Int. 2015;2015:851823.
Futyma K, Nowakowski Ł, Gałczyński K, Miotła P, Rechberger T. Nonabsorbable urethral bulking agent—clinical effectiveness and late complications rates in the treatment of recurrent stress urinary incontinence after 2 years of follow-up. Eur J Obstet Gynecol Reprod Biol. 2016;207:68–72.
Zajda J, Farag F. Urolastic-a new bulking agent for the treatment of women with stress urinary incontinence: outcome of 12 months follow up. Adv Urol 2013. 2013;724082:1–5.
Chan WKW, Chu PSK. Bulking agents in the management of urinary incontinence: dead or alive? Curr Bladder Dysfunct Rep. 2017;12(3):195–200.
Kasi AD, Pergialiotis V, Perrea DN, Khunda A, Doumouchtsis SK. Polyacrylamide hydrogel (Bulkamid®) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016;27(3):367–75.
Siddiqui ZA, Abboudi H, Crawford R, Shah S. Intraurethral bulking agents for the management of female stress urinary incontinence: a systematic review. Int Urogynecol J. 2017;28(29):1275–84.
Ghoniem G, Corcos J, Comiter C, Westney OL, Herschorn S. Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J Urol. 2010;183(4):1444–9.
Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012;(2): CD003881.
Acknowledgements
The PhD School of the Biomedical Sciences, Gender Medicine: Men, Women, and Children, Sassari University, Italy, supported the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None. The authors alone are responsible for the content and writing of the paper.
Additional information
Content
A meta-analysis was carried out on studies on the Urolastic® injectable elastomer prosthesis
Rights and permissions
About this article
Cite this article
Capobianco, G., Azzena, A., Saderi, L. et al. Urolastic®, a new bulking agent for treatment of stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J 29, 1239–1247 (2018). https://doi.org/10.1007/s00192-018-3703-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3703-6