Abstract
Introduction and hypothesis
Alternative approaches to reinforce the native tissue in patients with pelvic organ prolapse (POP) are needed to improve surgical outcome. Our aims were to develop a weakened abdominal wall in a rat model to mimic the weakened vaginal wall in women with POP and then evaluate the regenerative potential of a quickly biodegradable synthetic scaffold, methoxypolyethylene glycol polylactic-co-glycolic acid (MPEG-PLGA), seeded with autologous muscle fiber fragments (MFFs) using this model.
Methods
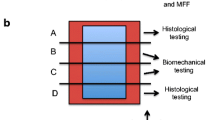
In an initial pilot study with 15 animals, significant weakening of the abdominal wall and a feasible technique was established by creating a partial defect with removal of one abdominal muscle layer. Subsequently, 18 rats were evenly divided into three groups: (1) unrepaired partial defect; (2) partial defect repaired with MPEG-PLGA; (3) partial defect repaired with MPEG-PLGA and MFFs labeled with PKH26-fluorescence dye. After 8 weeks, we performed histopathological and immunohistochemical testing, fluorescence analysis, and uniaxial biomechanical testing.
Results
Both macroscopically and microscopically, the MPEG-PLGA scaffold was fully degraded, with no signs of an inflammatory or foreign-body response. PKH26-positive cells were found in all animals from the group with added MFFs. Analysis of variance (ANOVA) showed a significant difference between groups with respect to load at failure (p = 0.028), and post hoc testing revealed that the group with MPEG-PLGA and MFFs showed a significantly higher strength than the group with MPEG-PLGA alone (p = 0.034).
Conclusion
Tissue-engineering with MFFs seeded on a scaffold of biodegradable MPEG-PLGA might be an interesting adjunct to future POP repair.



Similar content being viewed by others
Abbreviations
- POP:
-
Pelvic organ prolapse
- MFFs:
-
Muscle fiber fragments
- MPEG-PLGA:
-
Methoxypolyethylene glycol polylactic-co-glycolic acid
- SD:
-
Standard deviation
References
Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–21.
Lysaght MJ, Hazlehurst AL. Tissue engineering: the end of the beginning. Tissue Eng. 2004;10:309–20.
Boennelycke M, Gras S, Lose G. Tissue engineering as a potential alternative or adjunct to surgical reconstruction in treating pelvic organ prolapse. Int Urogynecol J. 2013;24:741–7.
Roman S, Mangera A, Osman NI, Bullock AJ, Chapple CR, MacNeil S. Developing a tissue engineered repair material for treatment of stress urinary incontinence and pelvic organ prolapse-which cell source? Neurourol Urodyn. 2014;33:531–7.
Chen B, Dave B. Challenges and future prospects for tissue engineering in female pelvic medicine and reconstructive surgery. Curr Urol Rep. 2014;15:425.
Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. 2013;13:1387–400.
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5.
Boennelycke M, Christensen L, Nielsen LF, Gräs S, Lose G. Fresh muscle fiber fragments on a scaffold in rats-a new concept in urogynecology? Am J Obstet Gynecol. 2011;205:235.e10–4.
Gräs S, Klarskov N, Lose G. Intraurethral injection of autologous minced skeletal muscle: a simple surgical treatment for stress urinary incontinence. J Urol. 2014;192:850–5.
Jangö H, Gräs S, Christensen L, Lose G. Muscle fragments on a scaffold in rats: a potential regenerative strategy in urogynecology. Int Urogynecol J. 2015;26:1843–51.
Chen B, Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol. 2011;186:1768–72.
Feola A, Duerr R, Moalli P, Abramowitch S. Changes in the rheological behavior of the vagina in women with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2013;24:1221–7.
Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–84.
Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport Sports Med Aust. 2014;17:346–50.
Feola A, Abramowitch S, Jallah Z, Stein S, Barone W, Palcsey S, et al. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG Int J Obstet Gynaecol. 2013;120:224–32.
Jones KA, Feola A, Meyn L, Abramowitch SD, Moalli PA. Tensile properties of commonly used prolapse meshes. Int Urogynecol J. 2009;20:847–53.
Corona BT, Garg K, Ward CL, McDaniel JS, Walters TJ, Rathbone CR. Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. AJP Cell Physiol. 2013;305:C761–75.
Garg K, Ward CL, Rathbone CR, Corona BT. Transplantation of devitalized muscle scaffolds is insufficient for appreciable de novo muscle fiber regeneration after volumetric muscle loss injury. Cell Tissue Res. 2014;358:857–73.
Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, Walters TJ. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials. 2013;34:3324–35. Elsevier Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclaimers
The Danish National Advanced Technology Foundation and the Nordic Urogynecological Association have supported this study. Coloplast A/S provided scaffolds and facilities for biomechanical and histological testing.
Conflicts of interest
G. Lose has received compensation as a consultant for Astellas. H. Jangö, S. Gräs, and L. Christensen have no conflicts of interest.
Additional information
Some preliminary results were presented at the 29th Bi-Annual Meeting of Nordic Urogynecological Association 29–31 January 2015, Stockholm, Sweden
Rights and permissions
About this article
Cite this article
Jangö, H., Gräs, S., Christensen, L. et al. Tissue-engineering with muscle fiber fragments improves the strength of a weak abdominal wall in rats. Int Urogynecol J 28, 223–229 (2017). https://doi.org/10.1007/s00192-016-3091-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-016-3091-8