Abstract
Introduction and hypothesis
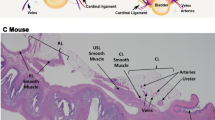
Pelvic floor muscles (PFM) are deleteriously affected by vaginal birth, which contributes to the development of pelvic floor disorders. To mechanistically link these events, experiments using animal models are required, as access to human PFM tissue is challenging. In choosing an animal model, a comparative study of PFM design is necessary, since gross anatomy alone is insufficient to guide the selection.
Methods
Human PFM architecture was measured using micromechanical dissection and then compared with mouse (n = 10), rat (n = 10), and rabbit (n = 10) using the Architectural Difference Index (ADI) (parameterizing a combined measure of sarcomere length-to-optimal-sarcomere ratio, fiber-to-muscle-length ratio, and fraction of total PFM mass and physiological cross-sectional area (PCSA) contributed by each muscle). Coccygeus (C), iliocaudalis (IC), and pubocaudalis (PC) were harvested and subjected to architectural measurements. Parameters within species were compared using repeated measures analysis of variance (ANOVA) with post hoc Tukey’s tests. The scaling relationships of PFM across species were quantified using least-squares regression of log-10-transformed variables.
Results
Based on the ADI, rat was found to be the most similar to humans (ADI = 2.5), followed by mouse (ADI = 3.3). When animals’ body mass was regressed against muscle mass, muscle length, fiber length, and PCSA scaling coefficients showed a negative allometric relationship or smaller increase than predicted by geometric scaling.
Conclusion
In terms of muscle design among commonly used laboratory animals, rat best approximates the human PFM, followed by mouse. Negative allometric scaling of PFM architectural parameters is likely due to the multifaceted function of these muscles.



Similar content being viewed by others
References
Subak LL et al (2001) Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol 98(4)
Kearney R et al (2010) Levator ani injury in primiparous women with forceps delivery for fetal distress, forceps for second stage arrest, and spontaneous delivery. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 111(1):19–22
DeLancey JOL et al (2007) Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 109(2, Part 1):295–302. doi:10.1097/01.AOG.0000250901.57095.ba
Otto LN et al (2002) The rhesus macaque as an animal model for pelvic organ prolapse. Am J Obstet Gynecol 186(3):416–421
Lieber RL, Fridén J (2000) Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 23(11):1647–1666
Powell PL et al (1984) Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol 57(6):1715–1721
Winters TM et al (2011) Whole muscle length-tension relationships are accurately modeled as scaled sarcomeres in rabbit hindlimb muscles. J Biomech 44(1):109–115
Lieber RL, Yeh Y, Baskin RJ (1984) Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter Biophysical Journal 45(5):1007–1016
Tuttle LJ et al (2013) Architectural design of the pelvic floor is consistent with muscle functional subspecialization. Int Urogynecol J
Gould SJ (1966) Allometry and size in ontogeny and phylogeny. Biol Rev Camb Philos Soc 41(4):587–640
Schmidt-Nielsen K (1975) Scaling in biology: the consequences of size. J Exp Biol 194:287–307
Alexander RM, Jayes AS, Maloiy GMO, Wathuta EM (1981) Allometry of the large muscles of mammals. J Zool 194:539–552
Burkholder TJ, Lieber RL (2001) Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204(Pt 9):1529–1536
Kawakami Y, Ichinose Y, Fukunaga T (1998) Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol 85(2):398–404
Ward SR, Lieber RL (2005) Density and hydration of fresh and fixed human skeletal muscle. J Biomech 38(11):2317–2320
Lieber RL, Brown CC (1992) Quantitative method for comparison of skeletal muscle architectural properties. J Biomech 25(5):557–560
Bremer RE et al (2003) Innervation of the levator ani and coccygeus muscles of the female rat. Anat Rec A: Discov Mol Cell Evol Biol 275(1):1031–1041
Martinez-Gomez M et al (1997) Striated muscles and scent glands associated with the vaginal tract of the rabbit. Anat Rec 247(4):486–495
Brown SH et al (2010) Architectural and morphological assessment of rat abdominal wall muscles: comparison for use as a human model. J Anat 217(3):196–202
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17(4):359–411
Lieber RL (1993) Skeletal muscle architecture: implications for muscle function and surgical tendon transfer. J of hand therapy : official journal of the American Society of Hand Therapists 6(2):105–113
Lieber RL (2008) Biology and mechanics of skeletal muscle: what hand surgeons need to know when tensioning a tendon transfer. The J of Hand Surgery 33(9):1655–1656
Feinberg AW et al (2012) Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 33(23):5732–5741
Couri BM et al (2012) Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol 7(3):249–260
Bracken JN et al (2011) Alterations in pelvic floor muscles and pelvic organ support by pregnancy and vaginal delivery in squirrel monkeys. Int Urogynecol J 22(9):1109–1116
Pierce LM et al (2007) Levator ani muscle and connective tissue changes associated with pelvic organ prolapse, parity, and aging in the squirrel monkey: a histologic study. Am J Obstet Gynecol 197(1):60.e1–60.e9
Mathewson MA et al (2013) Comparison of rotator cuff muscle architecture among humans and selected vertebrate species. J Exp Biol
Alperin M et al (2010) Collagen scaffold: a treatment for simulated maternal birth injury in the rat model. Am J Obstet Gynecol 202(6):589.e1–589.e8
Alperin M et al (2010) Pregnancy- and delivery-induced biomechanical changes in rat vagina persist postpartum. Int Urogynecol J 21(9):1169–1174
Lin AS et al (1998) Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology 52(1):143–151
Funding
The authors gratefully acknowledge funding by NIH grants K12 HD001259 and R24 HD050837.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alperin, M., Tuttle, L.J., Conner, B.R. et al. Comparison of pelvic muscle architecture between humans and commonly used laboratory species. Int Urogynecol J 25, 1507–1515 (2014). https://doi.org/10.1007/s00192-014-2423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-014-2423-9